
Non-invasive ventilation for acute respiratory failure: pressure support ventilation vs. pressure-controlled ventilation
Introduction
Non-invasive ventilation (NIV) has been widely used as the first-line strategy for improving oxygenation and ventilation in patients with acute respiratory failure (ARF) in the intensive care unit (ICU), with various applications in clinical practice, such as to facilitate early weaning from invasive mechanical ventilation (IMV) (1,2), for respiratory support after surgery (3), during certain procedures (4) or as palliative therapy (5). Its beneficial effects have been demonstrated in patients with acute hypercapnic respiratory failure (AHRF) (6), but questions remain regarding its efficacy for hypoxaemic respiratory failure, particularly de novo respiratory failure (7,8).
An NIV machine can deliver either pressure- or volume-targeted ventilation (9). Compared to volume-targeted ventilation, pressure-targeted ventilation has advantages of compensating for leaks and limiting high airway pressure (2,10-12). However, the most appropriate mode for NIV has not been clearly established. Pressure support ventilation (PSV), a flow-cycled mode, is widely used in many centres for AHRF. However, in the presence of large leaks, it can prolong inspiratory time, resulting in patient-ventilator asynchrony (12). By contrast, with assisted pressure-controlled ventilation (APCV), a time-cycled mode, the maximum inspiratory time can be set, theoretically achieving effective CO2 removal and promoting better synchrony.
Only a few studies have reported comparisons of the two pressure-targeted NIV modes, and no significant differences were detected (12). This multicentre prospective observational study was performed to compare the two modes (PSV vs. PCV) in terms of NIV success and complication rates in the ICU setting.
Methods
Study population
This was a prospective multicentre observational study conducted in 20 ICUs of university-affiliated hospitals in South Korea from June 1, 2017, to February 28, 2018, and some of the data were previously reported (13). Adult patients (age >18 years) who were admitted to the ICUs and received NIV treatment (at least 2 h) for ARF were prospectively enrolled in the study. Among the indications for NIV, AHRF indicates respiratory failure in patients with chronic lung disease (obstructive or restrictive), and de novo ARF usually indicates respiratory failure in patients without chronic lung disease, mostly those with hypoxaemic respiratory failure, such as pneumonia, post-operative respiratory failure, sepsis or acute respiratory distress syndrome (ARDS) (14,15). Among all patients initially included in the study, we excluded patients with do-not-resuscitate (DNR) orders and finally selected only patients treated with PCV or PSV mode.
The ethics committees of all participating hospitals approved this study, as did the Hallym University Institutional Review Board (approval no. 2017-I044). Informed consent was obtained from all enrolled patients or their legal surrogates.
Data collection and outcomes
We collected patient demographic information and the following data: comorbidities, underlying lung diseases, primary indications for NIV, and Richmond Agitation Sedation Score (RASS) and Sequential Organ Failure Assessment (SOFA) immediately before starting NIV. We also assessed the results of arterial blood gas analyses as well as vital signs before and 2 h after commencement of NIV. We investigated the type of NIV machine [i.e., invasive mechanical ventilator (IMV) with NIV module, IMV without NIV module, or home mechanical ventilator (MV)] and the interface (i.e., oronasal, nasal or total facial mask, helmet). In addition, the NIV settings [fractional inspired oxygen (FiO2), inspiratory positive airway pressure (IPAP), expiratory positive airway pressure (EPAP), and tidal volume] and their median durations (hours/day) were also investigated.
Treatment success and failure rates, complications from NIV treatment and ICU and in-hospital mortality rates were investigated as patient outcomes. Treatment success indicated successful weaning from NIV (i.e., a minimal duration of 24 h without NIV); the overall duration of NIV was determined by the physician in charge based on clinical improvement and arterial blood gas results. Treatment failure was defined as: (I) endotracheal intubation and invasive MV; (II) tracheostomy; and (III) hopeless discharge with NIV device. The following criteria were used for endotracheal intubation: (I) loss of consciousness; (II) hemodynamic instability (i.e., systolic blood pressure <90 mmHg despite fluid or need for vasopressors); and (III) worsening of respiratory distress under NIV (i.e., respiratory rate >40 breaths/minute or SpO2 remaining below 90% despite FiO2 100%). Patients who died within 24 h of NIV weaning were also classified as NIV failures. Large leaks were defined as leak flow >60 L/min or when the attending physician considered it too large to allow the treatment to continue.
The primary outcomes in this study were comparisons of NIV success and complication rates between patients treated with PCV vs. PSV mode. Secondary outcomes were risk factors for NIV success and in-hospital mortality rates.
Statistical analyses
All categorical variables are presented as numbers with percentages, and all continuous variables are presented as medians with interquartile ranges. Mann-Whitney U test was used to compare continuous variables, and the chi-square or Fisher’s exact test was used to compare categorical variables. Logistic regression analyses were performed using covariates with P<0.10 in univariate analyses to identify independent factors for NIV success (and in-hospital mortality); we employed a backward stepwise selection method based on the likelihood ratio. To reduce selection bias and confounding effects, we also performed matched analysis. We matched the patients with nearest-neighbor matching method, in a 1:1 ratio (PCV vs. PSV), for severity and other baseline variables which were significantly different between the two groups. All statistical analyses were performed using R, version 3.3.1, (R Foundation Inc.; http://cran.r-project.org/). In all analyses, P<0.05 was taken to indicate statistical significance.
Results
Study population
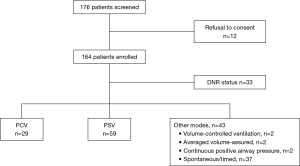
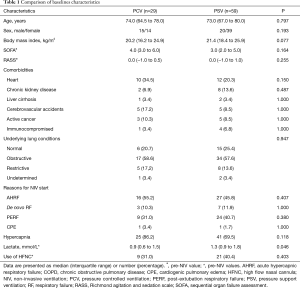
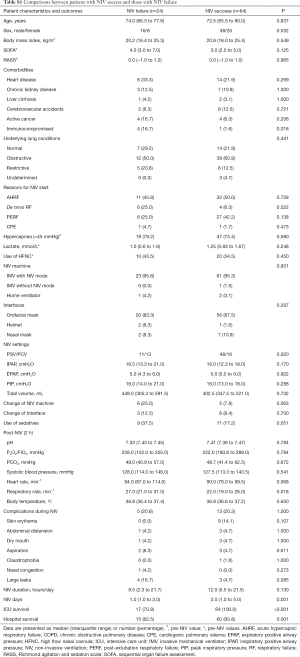
During the study period, 176 patients with ARF receiving NIV in the ICUs were initially included. After excluding 88 patients (withholding of consent, n=12; DNR order, n=33; other NIV modes, n=43), 88 patients (PCV, n=29; PSV, n=59) were included (Figure 1). The median age of the study population was 73.0 years (66.3–79.0 years), and 39 (44.3%) patients were female (Table 1). Eight patients had active cancer and five were in an immunocompromised state other than cancer. As the primary indication for NIV, AHRF was the most common (n=43, 48.9%), followed by post-extubation respiratory failure (PERF, n=33) and de novo ARF (n=10). Obstructive lung disease was the most common underlying lung disease (n=51), and a total of 20 patients (22.7%) received at least one sedative, among which remifentanil was the most commonly used (n=12).
Full table
NIV machine and interfaces
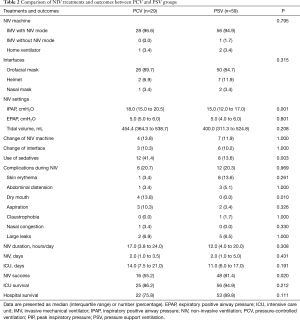
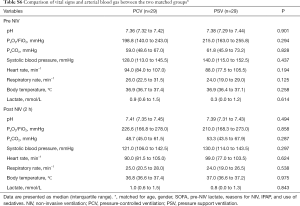
IMV with NIV mode was used in 95.5% of the patients, IMV without NIV mode was used in one patient and a home ventilator was used in three patients (Table 2). The orofacial mask was the most common interface used (86.4%), and there were no significant differences in NIV machine or interfaces used between the PCV and PSV groups. NIV time (h/day) and NIV days were also similar between the two groups. However, during NIV treatment, more patients in the PCV group used sedatives than in the PSV group [12 (41.4%) vs. 8 (13.6%), respectively, P=0.003]. Applied inspiratory positive airway pressure (IPAP) was significantly higher in the PCV group than the PSV group [18.0 cmH2O (15.0–20.5 cmH2O) vs. 15.0 cmH2O (12.0–17.0 cmH2O), respectively, P=0.001]. There were no differences in arterial blood gas parameters (in both pre-NIV and post-2-h-NIV periods) between the two groups. However, pre-NIV respiratory rate tended to be higher in the PCV group (Table 3). With regard to the differences (i.e., delta values) between pre-NIV and post-2-h-NIV periods, the arterial blood gas parameters and vital signs were similar between the PCV and PSV groups (data not shown).
Full table

Full table
NIV outcomes and complications
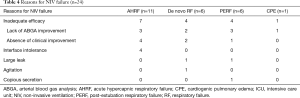
NIV success was achieved in 64 (72.7%) patients; among them, 15 patients were weaned off NIV in the general ward. The PSV group had a higher NIV success rate than the PCV group (81.4% vs. 55.2%, P=0.020; Table 2). Among 24 (27.3%) patients with NIV failure, 20 were intubated and received invasive ventilation, and four underwent tracheostomy. The most common reasons for NIV failure were the lack of arterial blood gas improvement (n=9) or absence of clinical improvement (n=7; Table 4). With regard to the primary indications for NIV, de novo ARF was more frequent in patients with NIV failure than in those with NIV success (25.0% vs. 6.3%, respectively, P=0.022; Table S1). A total of 18 (20.5%) patients experienced complications associated with NIV treatment; skin erythema was the most common and large leaks were reported in seven patients (Table 2). However, dry mouth was more frequent in the PCV group than the PSV group (13.8% vs. 0.0%, respectively, P=0.010).
Full table
Full table
Risk factors for NIV outcomes
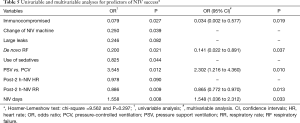
In univariate analyses, nine variables were associated with NIV success (P<0.10; Table S1). In multivariate analyses, five variables (immunocompromised condition, de novo respiratory failure, post-2-h-NIV respiratory rate, NIV days, and PSV mode) were significantly associated with NIV success, and PSV mode showed an OR of 2.302 (95% CI, 1.216–4.360) for NIV success (Table 5). However, 75 (85.2%) patients survived to discharge (Table S2); 15 (62.5%) patients in the NIV failure group (n=24) survived. In multivariate analyses, PERF and low post-2-h-NIV heart rate were significantly associated with survival until discharge (Table S3).
Full table

Full table

Full table
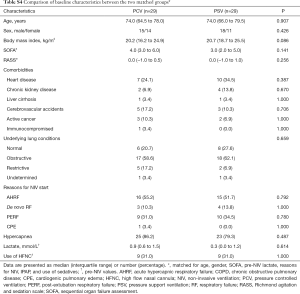
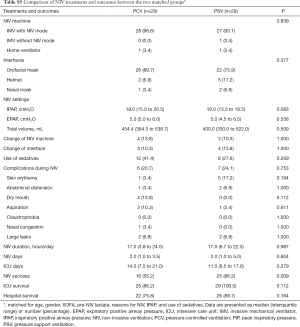
For the analysis of matched data, the two groups (PSV vs. PCV) were matched for age, gender, SOFA, pre-NIV lactate, reasons for NIV, IPAP levels, and use of sedatives (i.e., 29 pairs). The baseline characteristics were well balanced between the two groups (Tables S4-S6). In the multivariate analysis, where seven variables were finally included, PSV mode was a significant factor for NIV success (OR, 4.080; 95% CI, 1.020–16.321; Table S7).
Full table
Full table
Full table

Full table
Discussion
This study yielded several interesting results. First, PSV mode was associated with the use of lower IPAP levels than PCV mode in patients receiving NIV for ARF in the ICU setting. Second, the frequency of sedative use and the occurrence of dry mouth were higher in the PCV group than the PSV group. Finally, the OR of NIV success in the PSV group was double that in the PCV group; this association remained significant in the matched cohort.
The present study was small sized and study population was heterogeneous; both NIV failure and mortality rates varied depending on the causes of ARF (i.e., AHRF, de novo ARF, PERF and cardiogenic pulmonary oedema; Table S2). However, to date, few studies have compared the two pressure-targeted modes in patients receiving NIV for ARF. Previously, NIV failure and mortality rates were reported to be higher in patients with de novo ARF (i.e., 37–51.6% and 28.2–35.8%, respectively) (16-18) than in those with AHRF, PERF or receiving NIV for facilitation of IMV weaning (6,19-25). Both NIV failure and mortality rates were less than 30% in the latter three groups. Hence, the NIV outcomes in our cohort seemed to be comparable to those in previous studies. In our cohort, however, the use of NIV for facilitation of IMV weaning was not identified as a separate category from the PERF group. Besides, the NIV success group included patients who were transferred to the general ward in a stable condition with the NIV device in place (n=15); all patients were ultimately weaned off NIV and afterwards, two died.
Patients treated with other modes, mostly Spontaneous/Time (S/T) mode, were excluded from the present study because the aim was to compare the PCV and PSV modes among patients with NIV treatment. However, the rate of NIV success was also significantly higher in patients with PSV mode than other modes [81.4% (48/59) vs. 62.8% (27/43), respectively, P=0.032]. These results suggest that NIV mode where the cycle variable depends on the patient’s inspiratory effort may be better or more suitable for patients with ARF in the ICU setting. However, Kirakli et al. reported that PCV mode may be more effective for eliminating CO2 compared to PSV mode and may be better tolerated in patients requiring high inspiratory flow rate in the presence of leaks (12). In the presence of large leaks, patients with PSV mode may experience difficulty terminating inspiratory phase, leading to patient-ventilator asynchrony. In the present study, although we did not obtain detailed data on the inspiratory times or air leaks, the frequency of large leaks was low in both groups (n=2 in PSV mode vs. n=5 in PCV mode). This may have mitigated the negative effects of PSV.
Interestingly, the level of IPAP was higher in the PCV group than the PSV group. Although data are not shown, IPAP was significantly correlated with pre-NIV PaCO2 (r=0.333 and P=0.002) and pre-NIV pH (r=−0.297 and P=0.005). Therefore, it is likely that patients with high PaCO2 were treated with a high level of IPAP using PCV mode. Patients with PCV mode required sedatives and experienced dry mouth more frequently, which may be explained by their high levels of IPAP. However, it should be noted that although the goal of NIV application is to increase alveolar ventilation leading to decreased work of breathing, the high pressure support levels (to increase alveolar ventilation) may not be useful (or may rather be harmful) because they are not associated with the recruitment of the poorly ventilated area (12,26).
The higher NIV success rate with PSV mode may have been due to better patient-ventilator synchrony compared to PCV mode. However, we do not have any specific data supporting the association. Instead, as initial SOFA score and pre-NIV PaCO2 were lower and pre-NIV PaO2/FiO2 was higher in the PSV group, it is possible that the lower disease severity influenced the lower level of IPAP and higher rate of NIV success (27). In addition, as mentioned above, the occurrence of large leaks, which can compromise patient-ventilator synchrony with PSV mode, was uncommon in our patients. However, importantly, some different baseline characteristics and the observational nature of our study suggest that our data were prone to have selection bias (or confounding effects). To control this effect, we matched patients for several baseline variables, including severity score, and found that the association of PSV mode with NIV success remained significant in the matched cohort. Nonetheless, considering the small sample size and potential confounders, there might be overfitting of the multivariate models.
The present study has some limitations. First, there may have been unintended bias in the results because our study was not randomised and sample size was small. Again, we cannot exclude confounding effects entirely. Second, we did not use a protocol driven algorithm for NIV treatment. Hence, the selection of mode or change of NIV machine was determined at the discretion of participating physicians, and the practice for NIV treatment varied among the participating hospitals. Third, a large number of patients who consented to the study were initially excluded, and there were multiple indications for NIV treatments (i.e., heterogeneity of study population). Fourth, uniquely, the variation of body mass index was smaller, compared to that of other studies (28-30), which could limit the generalisability of the study. This must be taken into consideration when interpreting our results. Fifth, despite the significant association with NIV success, the PSV mode was not associated with hospital survival. Although the hospital (and ICU) survival rate was numerically lower in PCV group vs. PSV group, further studies with a larger sample size will be needed to clarify this. Sixth, for the majority of patients (96.6%), an IMV machine was used for NIV instead of a dedicated NIV machine, and in particular, four patients used dissimilar NIV machine. Finally, we could not investigate the long-term outcomes among patients. However, to date, there have been few studies comparing the two pressure-targeted modes among patients receiving NIV for ARF. Hence, our results are meaningful and may prompt future studies on interesting topics. For example, it may be possible to find subgroups that are best fit for PSV mode (or PCV mode) through future well-designed studies.
Conclusions
In conclusion, we found that PSV mode was significantly associated with higher rate of NIV success than PCV mode in the ICU setting, particularly when the occurrence of large leaks is not a major concern. However, the mode was not associated with better hospital survival. Future large-scale, protocol-driven, randomised controlled trials are needed to confirm our results.
Acknowledgments
The authors sincerely thank the following investigators of Korean NIV study group for the participation in this study: Jin Woo Kim (The Catholic University of Korea, Uijeongbu St. Mary’s Hospital), Jong Hoo Lee (Jeju National University Hospital), Tae Oak Kim (Chonnam National University Hospital), Seung Yong Park (Chonbuk National University Hospital), M.D Won-Il Choi (Kyeimyung University Dongsan Hospital), and Yun Su Sim (Hallym University Kangnam Sacred Heart Hospital).
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.27). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The ethics committees from all participating hospitals approved this study, as did the Hallym University Institutional Review Board (approval no. 2017-I044). Informed consent was obtained from all enrolled patients or their legal surrogates.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ornico SR, Lobo SM, Sanches HS, et al. Non-invasive ventilation immediately after extubation improves weaning outcome after acute respiratory failure: a randomized controlled trial. Crit Care 2013;17:R39. [Crossref] [PubMed]
- Vitacca M, Rubini F, Foglio K, et al. Non-invasive modalities of positive pressure ventilation improve the outcome of acute exacerbations in COLD patients. Intensive Care Med 1993;19:450-5. [Crossref] [PubMed]
- Zarbock A, Mueller E, Netzer S, et al. Prophylactic nasal continuous positive airway pressure following cardiac surgery protects from postoperative pulmonary complications: a prospective, randomized, controlled trial in 500 patients. Chest 2009;135:1252-9. [Crossref] [PubMed]
- Murgu SD, Pecson J, Colt HG. Bronchoscopy during non-invasive ventilation: indications and technique. Respir Care 2010;55:595-600. [PubMed]
- Nava S, Ferrer M, Esquinas A, et al. Palliative use of non-invasive ventilation in end-of-life patients with solid tumours: a randomised feasibility trial. Lancet Oncol 2013;14:219-27. [Crossref] [PubMed]
- Brochard L, Mancebo J, Wysocki M, et al. Non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995;333:817-22. [Crossref] [PubMed]
- Demoule A, Girou E, Richard JC, et al. Benefits and risks of success or failure of non-invasive ventilation. Intensive Care Med 2006;32:1756-65. [Crossref] [PubMed]
- Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: non-invasive ventilation for acute respiratory failure. Eur Respir J 2017;50:1602426. [Crossref] [PubMed]
- Rabec C, Rodenstein D, Leger P, et al. Ventilator modes and settings during non-invasive ventilation: effects on respiratory events and implications for their identification. Thorax 2011;66:170-8. [Crossref] [PubMed]
- Cao Z, Luo Z, Hou A, et al. Volume-targeted vs. pressure-limited non-invasive ventilation in subjects with acute hypercapnic respiratory failure: a multicenter randomized controlled trial. Respir Care 2016;61:1440-50. [Crossref] [PubMed]
- Girault C, Richard JC, Chevron V, et al. Comparative physiologic effects of non-invasive assist-control and pressure support ventilation in acute hypercapnic respiratory failure. Chest 1997;111:1639-48. [Crossref] [PubMed]
- Kirakli C, Cerci T, Ucar ZZ, et al. Non-invasive assisted pressure-controlled ventilation: as effective as pressure support ventilation in chronic obstructive pulmonary disease? Respiration 2008;75:402-10. [Crossref] [PubMed]
- Nam H, Cho JH, Choi EY, et al. Current status of non-invasive ventilation use in Korean intensive care units: a prospective multicenter observational study. Tuberc Respir Dis 2019;82:242-50. [Crossref] [PubMed]
- Demoule A, Chevret S, Carlucci A, et al. Changing use of non-invasive ventilation in critically ill patients: trends over 15 years in francophone countries. Intensive Care Med 2016;42:82-92. [Crossref] [PubMed]
- Demoule A, Girou E, Richard JC, et al. Increased use of non-invasive ventilation in French intensive care units. Intensive Care Med 2006;32:1747-55. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Non-invasive ventilation of patients with acute respiratory distress syndrome. Insights from the LUNG SAFE study. Am J Respir Crit Care Med 2017;195:67-77. [Crossref] [PubMed]
- Carteaux G, Millan-Guilarte T, De Prost N, et al. Failure of non-invasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. Crit Care Med 2016;44:282-90. [Crossref] [PubMed]
- Frat JP, Ragot S, Coudroy R, et al. Predictors of intubation in patients with acute hypoxemic respiratory failure treated with a non-invasive oxygenation strategy. Crit Care Med 2018;46:208-15. [Crossref] [PubMed]
- Auriant I, Jallot A, Herve P, et al. Non-invasive ventilation reduces mortality in acute respiratory failure following lung resection. Am J Respir Crit Care Med 2001;164:1231-5. [Crossref] [PubMed]
- Ferrer M, Valencia M, Nicolas JM, et al. Early non-invasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med 2006;173:164-70. [Crossref] [PubMed]
- Huang HW, Sun XM, Shi ZH, et al. Effect of high-flow nasal cannula oxygen therapy vs. conventional oxygen therapy and non-invasive ventilation on reintubation rate in adult patients after extubation: a systematic review and meta-analysis of randomized controlled trials. J Intensive Care Med 2018;33:609-23. [Crossref] [PubMed]
- Jaber S, Lescot T, Futier E, et al. Effect of non-invasive ventilation on tracheal reintubation among patients with hypoxemic respiratory failure following abdominal surgery: a randomized clinical trial. JAMA 2016;315:1345-53. [Crossref] [PubMed]
- Nava S, Gregoretti C, Fanfulla F, et al. Non-invasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med 2005;33:2465-70. [Crossref] [PubMed]
- Osadnik CR, Tee VS, Carson-Chahhoud KV, et al. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017;7:CD004104. [PubMed]
- Vargas F, Clavel M, Sanchez-Verlan P, et al. Intermittent non-invasive ventilation after extubation in patients with chronic respiratory disorders: a multicenter randomized controlled trial (VHYPER). Intensive Care Med 2017;43:1626-36. [Crossref] [PubMed]
- Diaz O, Iglesia R, Ferrer M, et al. Effects of non-invasive ventilation on pulmonary gas exchange and hemodynamics during acute hypercapnic exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997;156:1840-5. [Crossref] [PubMed]
- Tucci MR, Costa EL, Nakamura MA, et al. Non-invasive ventilation for acute respiratory distress syndrome: the importance of ventilator settings. J Thorac Dis 2016;8:e982-6. [Crossref] [PubMed]
- Patel BK, Wolfe KS, Pohlman AS, et al. Effect of Non-invasive Ventilation Delivered by Helmet vs. Face Mask on the Rate of Endotracheal Intubation in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2016;315:2435-41. [Crossref] [PubMed]
- Perkins GD, Mistry D, Gates S, et al. Effect of Protocolized Weaning With Early Extubation to Non-invasive Ventilation vs Invasive Weaning on Time to Liberation From Mechanical Ventilation Among Patients With Respiratory Failure: The Breathe Randomized Clinical Trial. JAMA 2018;320:1881-8. [Crossref] [PubMed]
- Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015;372:2185-96. [Crossref] [PubMed]


