
Advances in immunotherapy for stage III non-small cell lung cancer: moving immune checkpoint inhibitors to the front lines concurrently with chemoradiotherapy?
Lung cancer is a leading cause of cancer-associated death worldwide. Non-small cell lung cancer (NSCLC) accounts for more than 80% of all lung cancer cases. Among all cases of NSCLC, approximately two-thirds are advanced and inoperable at the time of diagnosis. Although concurrent chemoradiotherapy (cCRT), the standard treatment for patients with inoperable, locally advanced NSCLC (1), is potentially curative, the 5-year overall survival (OS) rate has reached a plateau around 20%, despite considerable attempts to improve treatment results by intensification of conformal radiation therapy and enhanced chemotherapy with induction or consolidation.
Recently, PD-1 or PD-L1 immune checkpoint inhibitors (ICIs) have shown exceptional efficacy in patients with advanced NSCLC, and preclinical and early phase clinical studies suggest that radiation therapy and chemotherapy work synergistically with ICIs to develop antitumor immunity (2,3), that has led to testing of ICIs in patients with stage III NSCLC through clinical trials. The randomized phase 3 PACIFIC study demonstrated outstanding increases in median progression-free survival (PFS) and OS after consolidation therapy using durvalumab, a PD-L1 inhibitor, after cCRT, compared with the outcomes of cCRT alone (4,5). The PACIFIC data encourage the usage of consolidative durvalumab immunotherapy after completion of cCRT in patients with stage III inoperable NSCLC based on an acceptable safety profile and significantly improved OS. The PACIFIC study raised query concerning the timing between cCRT and ICIs therapy. Additional durvalumab decreased progression, regardless of when it was started, but greater decrement was observed if durvalumab was started within 2 weeks of radiation therapy [hazard ratio (HR) =0.39; 95% confidence interval (CI) =0.26–0.58] instead of >2 weeks after radiation therapy (HR =0.63; 95% CI =0.49–0.80), implying a critical window for initiating PD-L1 blockade. This confirms the findings of reported studies that recognized enhanced results in patients treated with concurrent immune therapy or in close proximity to radiotherapy (6). Additionally, considering the potential of chemotherapy to synergize with ICIs, as shown by the KEYNOTE-021 trial (7), adding platinum-based chemotherapy to immunotherapy could enhance responses. Recently, trials involving ICIs initiated immunotherapy at various time points, and their collective outcomes may clarify the ideal timing between cCRT and ICIs.
Jabbour et al. conducted a phase 1, nonrandomized, controlled trial of cCRT [weekly carboplatin and paclitaxel plus 60 Gy of radiation (2 Gy per day)] with concurrent PD-1 blockade using pembrolizumab (8). The evaluated dose cohorts included full-dose pembrolizumab (200 mg intravenously every 3 weeks) 2–6 weeks after cCRT (cohort 1), reduced-dose pembrolizumab (100 mg intravenously every 3 weeks) starting on day 29 of cCRT (cohort 2), full-dose pembrolizumab starting on day 29 of cCRT (cohort 3), reduced-dose pembrolizumab starting on day 1 of cCRT (cohort 4), and full-dose pembrolizumab starting on day 1 of cCRT (cohort 5). A safety expansion cohort of six patients was planned based on the maximum tolerated dose of pembrolizumab. Dose-limiting toxicity was defined as pneumonitis of at least grade 4 during the first cycle of pembrolizumab treatment. Among the 21 patients included in the analysis, no dose-limiting toxic effects were observed in any cohort. One case of grade 5 pneumonitis occurred in the safety expansion cohort with the cohort 5 regimen. Immune-related adverse events of at least grade 3 occurred in four patients (18%). PD-1 inhibition and cCRT for stage III NSCLC was tolerable with an 18% rate of grade 3 or greater immune-related adverse events, including grades 3 and 5 pneumonitis, grade 3 interstitial nephritis, and type 1 diabetes. The overall rate of pneumonitis of at least grade 2 was 33%, and in the PACIFIC study, any-grade pneumonitis and radiation pneumonitis occurred in 33.9% and 24.8% of patients, respectively. The PACIFIC study enrolled patients after cCRT and excluded those with preexisting pneumonitis of at least grade 2 caused by cCRT, in contrast to the study by Jabbour et al., in which patients were registered before cCRT. In this study, grade 3 pneumonitis and grade 5 pneumonitis occurred in one patient each (5%). In the PACIFIC study, the percentages of grades 3 or 4 pneumonitis and radiation-induced pneumonitis were 3.4% and 2.6%, respectively.
In patients who had been treated at least a dose of pembrolizumab (n=21), the median PFS was 18.7 months (95% CI =11.8–29.4) and the 6- and 12-month PFS rates were 81.0% (95% CI =64.1–97.7) and 69.7% (95% CI =49.3–90.2), respectively. The median PFS for patients who received at least two doses of pembrolizumab (n=19) was 21.0 months (95% CI =15.3 to infinity). The results suggested that combined treatment with PD-1 inhibitors and cCRT for stage III NSCLC is tolerable, with promising a PFS rate of 69.7% at 12 months. Although they observed an increased rate of pneumonitis, this adverse event responded to high-dose corticosteroid treatment in most patients. Adding pembrolizumab to cCRT in the definitive management of locally advanced NSCLC is feasible and generally well tolerated. They suggested that first-line therapy with PD-1 inhibition and cCRT for stage III NSCLC appears tolerable and suitable for evaluation in phase 2 and 3 clinical trials.
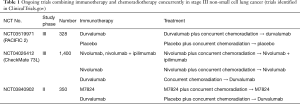
According to the phase 1 trial results, adding concurrent pembrolizumab to cCRT may represent a candidate first-line treatment regimen for stage III NSCLC. There is an ongoing phase 2 trial of pembrolizumab in combination with cCRT (9). In addition, two studies, DETERRED (NCT02525757) and NICOLAS (NCT02434081), assessed atezolizumab or nivolumab administration concurrently with cCRT (10,11). A phase 2 trial (WJOG11619L) investigating the effect and safety of durvalumab with concurrent radiation and a phase 2 study (TORG1937) evaluating those of durvalumab provided immediately after the completion of cCRT are both in progress in Japan, but their findings will require confirmation through randomized trials. A number of randomized trials in NSCLC are currently in the planning phase or actively recruiting patients to assess the efficacy of radiation plus concurrent immunotherapy. The trials, described in ClinicalTrials.gov, are presented in Table 1. The PACIFIC 2 study (NCT03519971), an international phase 3 trial, is currently investigating the efficacy and safety of durvalumab administered concurrently with platinum-based cCRT in patients with inoperable stage 3 NSCLC. Furthermore, CheckMate 73L, a randomized phase 3 trial comparing nivolumab plus cCRT followed by nivolumab/ipilimumab or nivolumab and cCRT followed by nivolumab versus cCRT followed by durvalumab in patients with previously untreated, inoperable NSCLC, is ongoing. This study’s primary purpose is to evaluate the potency of nivolumab plus cCRT followed by nivolumab plus ipilimumab versus cCRT followed by durvalumab in patients with inoperable stage III NSCLC. A double-blind, randomized study of M7824 with cCRT followed by M7824 versus cCRT plus placebo followed by durvalumab in patients with inoperable stage III NSCLC is also ongoing. M7824 is a bifunctional fusion protein comprising a monoclonal antibody against PD-L1 fused to the extracellular domain of transforming growth factor-β (TGF-β) receptor II that works as a trap for all three TGF-β isoforms. Reportedly, M7824 specifically, simultaneously, and efficiently binds TGF-β and PD-L1 (12); concurrent blocking of the TGF-β and PD-L1 pathways by M7824 demonstrates effective and superior antitumor activity relative to monotherapies; and primary resistance to ICIs therapy is overcome by the selective inhibition of TGF-β1 activation through the alteration of the tumor immune landscape (13).
Full table
How should oncologists select from several available ICIs and cCRT combination regimens in clinical trials? So far, there is no evidence to provide responses to these questions because no comparative studies combining ICIs and cCRT have been conducted and some issues remain to be addressed. The first issue is regarding whether the antitumor effects and safety of PD-1 or PD-L1 inhibitors are similar. The selection of a proper ICI among PD-L1, PD-1, and cytotoxic T-lymphocyte antigen 4 inhibitors for use with cCRT may be important. The second issue is when to initiate radiotherapy. The optimal timing of ICI therapy relative to cCRT remains to be investigated. Other questions include proper selection of the combination chemoradiation regimens and the optimal ICI modality for concurrent therapy with cCRT. To answer the questions, optimal treatment strategies for stage III NSCLC is under investigation in several trials. In addition, some adequate biomarkers for identification of the subset of patients with stage III NSCLC who may most benefit from ICI therapy combined with cCRT are necessary. Depending on the outcomes of the ongoing studies, new combination treatments involving cCRT and ICIs will be developed, although further investigations will be needed to preserve efficacy and reduce toxicity.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1672). TH has received grants and personal fees from AstraZeneca; Ono Pharmaceutical Co., Ltd.; Chugai Pharmaceutical Co., Ltd.; Bristol-Meyers Squibb; and MSD. TY has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [Crossref] [PubMed]
- Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687-95. [Crossref] [PubMed]
- Dovedi SJ, Cheadle EJ, Popple AL, et al. Fractionated Radiation Therapy Stimulates Antitumor Immunity Mediated by Both Resident and Infiltrating Polyclonal T-cell Populations when Combined with PD-1 Blockade. Clin Cancer Res 2017;23:5514-26. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Samstein R, Rimner A, Barker CA, et al. Combined immune checkpoint blockade and radiation therapy: timing and dose fractionation associated with greatest survival duration among over 750 treated patients. Int J Rad Oncol Biol Phys 2017;99:S129-30. [Crossref]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Jabbour SK, Berman AT, Decker RH, et al. Phase 1 Trial of Pembrolizumab Administered Concurrently With Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer: A Nonrandomized Controlled Trial. JAMA Oncol 2020;6:1-8. [Crossref] [PubMed]
- ClinicalTrials.gov. A trial of pembrolizumab in combination with chemotherapy and radiotherapy in stage III NSCLC (KEYNOTE-799, MK-3475-799). NCT03631784. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03631784, accessed April 1, 2019.
- Lin S, Lin X, Clay D, et al. OA01.06 DETERRED: phase II trial combining atezolizumab concurrently with chemoradiation therapy in locally advanced non-small cell lung Cancer J Thorac Oncol 2018;13:S320-S321. [Crossref]
- Peters S, Felip E, Dafni U, et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer-The ETOP NICOLAS trial. Lung Cancer 2019;133:83-7. [Crossref] [PubMed]
- Lan Y, Zhang D, Xu C, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med 2018. [Crossref] [PubMed]
- Martin CJ, Datta A, Littlefield C, et al. Selective inhibition of TGFβ1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci Transl Med 2020. [Crossref] [PubMed]


