
DACT2 regulates structural and electrical atrial remodeling in atrial fibrillation
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and the cause of considerable morbidity, mortality and health related expenditures (1). Effective therapy and prevention are important for controlling AF-related morbidity and mortality. However, the medical interventions that may be used for the treatment of AF are limited due to a lack of understanding of the mechanisms underlying the disease.
AF is the result of a complex continuum of predisposing factors. AF has been suggested to be primarily an electrical disease induced by disturbances in ionic currents and the genetic causes of these types of electrical disturbance are becoming increasingly recognized (2). In addition to electrical remodeling, structural remodeling is a very important event that contributes to AF development (3). Moreover, AF itself can induce electrical and structural changes in the atrium, thereby facilitating its persistence (4,5). Thus, the complexity of the etiology of atrial electrical dysfunction and the underlying histological alterations have prevented definitive elucidation.
DACT2, a member of the dapper protein family, has been reported to function not only as an antagonist of TGF-β/Nodal signaling (6-11) but also as an epigenetic regulator of Wnt signaling (12-15). DACT2 can inhibit TGF-β signaling by promoting lysosomal degradation of TGF-β receptors (7,8). Moreover, DACT2 could directly disrupt the formation of the β-catenin-LEF1 complex in the nucleus and restore the junctional localization of E-cadherin-β-catenin complexes in the cytoplasm (12). Both the TGF-β signaling and Wnt signaling pathways are key mediators of AF (5,16). Thus, it has been hypothesized that DACT2 may play a crucial role in AF. However, little is known regarding this topic.
In the present study, we investigated the relationships between DACT2 expression and AF involved valvular heart disease. Then, the levels of AF-related genes and signaling pathway activation were detected after DACT2 expression in primary atrial fibroblasts and the HL-1 cell line, which is a unique in vitro model used for the study of mammalian atrial myocyte ion channel regulation and functional expression.
Methods
This study was approved by the Human Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University and complied with the principles governing the use of human tissues that are outlined in the Declaration of Helsinki. Informed consent was obtained before participation in the study.
Human tissue preparation
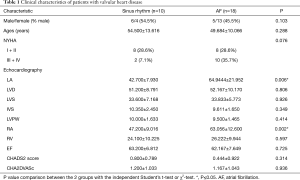
Tissue samples from the right atrial appendage were obtained from 28 patients with valvular heart disease. All of these patients had undergone valve replacement surgery. Ten patients, who constituted the sinus rhythm (SR) group, did not have a history of AF, and eighteen patients, who constituted the AF group, had documented arrhythmias from which they had suffered for more than six months before undergoing surgery. The above described tissue samples were obtained at the time of valve replacement surgery, and were immediately fixed in 4% paraformaldehyde (PFA). The diagnosis of AF was made based on patient medical records and 12-lead electrocardiogram (ECG) findings. Preoperative functional statuses were recorded in accordance with the New York Heart Association (NYHA) classification. Patient data are summarized in Table 1.
Full table
Immunohistochemical staining
All the samples were fixed in 4% PFA, embedded in paraffin and stained with hematoxylin and eosin for routine histological examination. Immunohistochemical staining was performed on 4-µm-thick paraffin sections. After deparaffinization and rehydration, all the sections were microwaved (10 min) in 0.01 mol/L sodium citrate buffer (pH 6.0) for antigen retrieval. To block endogenous peroxidase activity, we incubated the sections with 10% normal goat serum in PBS for 15 min at room temperature. Then, all the sections were incubated with a rabbit polyclonal antibody against DACT2 (1:100; Abcam, Cambridge, UK) overnight at 4 °C. The slides were subsequently treated with the SuperPic Ture Polymer Detection Kit and Liquid DAB Substrate Kit (Zymed/Invitrogen, San Francisco, USA), counterstained with hematoxylin, dehydrated, and mounted.
Masson’s trichrome staining
The sections were stained with Masson’s trichrome stain for fibrosis quantification. For Masson’s trichrome staining, the slices were dewaxed with xylol (2 dewaxing steps lasting 2 min each, followed by soaking in a series of graded alcohols with, concentrations ranging from 95% to 99%). All the slices were then washed in distilled water and placed in a hematoxylin solution for 3 min, after which a color change was induced with lithium carbonate. The slices were subsequently washed in pure water and subjected to Ponceau red staining (in an oven at 30 °C and 45 kW for 20 sec). The slices were then placed in acidic water and phosphomolybdic acid for 1 min before being labeled with a green fluorescent marker and washed with acidic water. Fibrosis severity was then assessed in each of the sections upon collection.
Immunostaining evaluation
Immunohistochemical staining was evaluated using Image-Pro Plus 6.0 software. Briefly, at least three fields with positive expression in a section of myocardial tissue were randomly selected, and then these positive regions were analyzed with Image-Pro Plus 6.0 to determine their integral optical density and area. The average optical density, which represented the expression intensity in the section was subsequently calculated. The average of the optical density values was determined to represent the expression intensity in the section. DACT2 expression was regarded as weak if the mean density was <0.004 and strong if the mean density was ≥0.004.
Fibrosis evaluation
Fibrosis severity was evaluated using Image-Pro Plus 6.0 software. At least three fields in a section of myocardial tissue were randomly selected after which the ratio of the fibrotic area to the total area of each selected field was calculated to assess fibrosis severity. The average ratio, which represented the severity of the fibrosis in the section of myocardial tissue, was subsequently determined. Fibrosis was regarded as weak if the mean ratio was <0.1, strong if the mean ratio was ≥0.1 and <0.3, and very strong if the mean ratio was ≥0.3.
Cell culture
The HL-1 cell line was obtained from EDM Millipore Corporation (Cat. SCC065). Cells were cultured in Claycomb medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin in flasks precoated with fibronectin and gelatin.
The primary rat atrial fibroblasts were harvested from adult male SD rats and cultured as reported previously (17). Briefly, atrial tissues were removed and washed with PBS. After enzymatic digestion with 0.1% collagenase II (Gibco, South Logan, UT, USA), atrial fibroblasts were cultured in Dulbecco’s modified Eagle medium (DMEM, Gibco) supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin and 10% fetal bovine serum (FBS, Gibco). After three passages, the cells were collected and passaged for further experiments.
All the cells were grown under a 5% CO2 atmosphere at 37 °C.
Adenovirus infection
The Ad-CMV-eGFP vector (GeneChem, Shanghai, China) was used for DACT2 (NM_214462.4) expression vector construction, while the con177 vector was used as a control. The adenoviral particles were produced by GeneChem. Cells were plated 24 hours before infection, incubated with fresh media containing the virus at the required multiplicity of infection (MOI) based on the cell concentration for 18 hours, washed, and maintained until harvesting. After infection 48 hours later, GFP-expressing cells were observed using a fluorescence microscope (Axio Observer Z1).
Real-time polymerase chain reaction (RT-PCR)
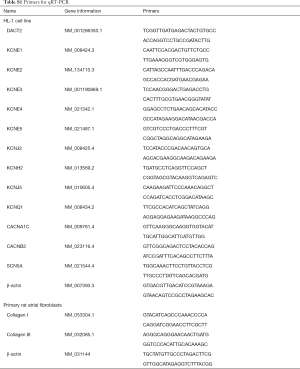
Total RNA was extracted from cells with TRIzol reagent (Invitrogen), and cDNA was synthesized using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). The PCR primer sequences are listed in Table S1. Quantitative PCR analysis was performed according to the manufacturer’s instructions with the FastStart Universal SYBR Green Master Mix (Rox) (Roche) by StepOnePlus (ABI). For analysis, the expression of target genes was normalized to that of β-actin. The gene expression level was determined using the delta Ct method (DCt), which is a variation of the Livak method, where DCt = Ct (reference gene) − Ct (target gene). The data analysis was based on results from three independent experiments.
Full table
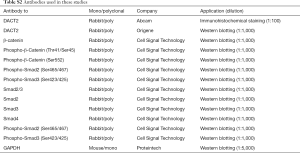
The technique used was described previously (6). Proteins were isolated from cells with lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) containing a protease inhibitor cocktail (Millipore, Billerica, MA, USA). The protein was subjected to SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA, USA). The primary antibodies used in this study are summarized in Table S2. Antigen-antibody complexes were detected by western blotting with luminol reagent (Santa Cruz Biotechnology).
Full table
Western blotting
Statistical analyses
The correlations between the immunohistochemical results and patient clinical variables were analyzed by χ2-tests. Continuous variables are represented as the mean ± SEM. Comparisons of continuous variables between groups were performed with Student’s t-test, and the correlations between DACT2 expression levels and fibrosis severity were assessed with the nonparametric Spearman rank correlation test. A P value less than 0.05 was considered statistically significant. All statistical analyses were performed with SPSS 19.0 software (version 19.0; SPSS, Inc., an IBM Company, Chicago, IL, USA).
Results
Patient characteristics
Table 1 shows the demographic data pertaining to the patients enrolled in this study. Patients with AF had greater mean left atrium (LA) (64.9444±21.952 vs. 42.700±7.930 mm, P=0.006) and right atrium (RA) dimensions (63.056±12.600 vs. 47.200±9.016 mm, P=0.003) than patients with SR. There were no significant differences between AF and SR patients with respect to the other clinical variables analyzed herein.
DACT2 expression in the myocardial tissue of patients with valvular disease
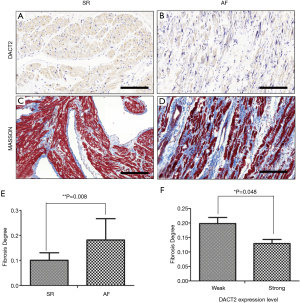
DACT2 expression was detected in the myocardial tissues of all patients with valvular disease who were enrolled in this study. Positive immunoreactivity for DACT2 was observed in the myocardial cell cytoplasm (Figure 1A,B), and strong DACT2 staining was noted in 17 (58.6%) of the 28 specimens analyzed in this investigation. The statistical analysis showed that DACT2 expression was significantly associated with AF and SR (Table 2, P=0.022). A higher percentage of patients with AF was noted in the weak DACT2 staining group than in the strong DACT2 staining group (Table 2, weak staining group vs. strong staining group: 32.1% vs. 28.6%). However, a significantly lower percentage of patients in SR was noted in the weak DACT2 group than in the strong DACT2 staining group (Table 2, weak staining group vs. strong staining group: 3.6% vs. 35.7%). These data suggest that DACT2 downregulation may be a risk factor for AF.
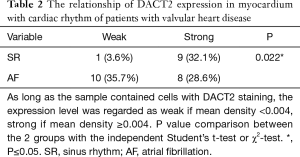
Full table
We also investigated the differences in patient clinical parameters between the two DACT2 expression groups in this study. We found that the RA dimension was significantly larger in the weak DACT2 expression group than in the strong DACT2 expression group (Table 3, weak expression group: 65.182±13.423 mm; strong expression group: 52.353±11.581; P=0.012). There were no significant differences between the two groups with respect to other parameters, such as the ejection fraction (EF), LA dimension, and LVD.

Full table
Correlation between DACT2 expression and fibrosis severity in patients with valvular disease
Fibrosis is crucial with respect to AF development because it plays an important role in arrhythmogenic structural remodeling (5,18). To investigate the relationship between DACT2 expression levels and fibrosis severity, we performed Masson’s trichrome staining to evaluate fibrosis severity (Figure 1C,D) and analyzed the statistical correlation between this parameter and DACT2 expression levels. We found that the fibrosis ratio in the AF group was much higher than that in the SR group (Figure 1E, AF group: 0.181±0.085; SR group: 0.101±0.029, P=0.008), a finding consistent with that of previous studies (3,19). However, the fibrosis ratio in the strong DACT2 expression group was significantly lower than that in the weak DACT2 expression group (Figure 1F, weak expression group: 0.198±0.091; strong expression group: 0.129±0.064, P=0.048). Further statistical analysis showed that a negative correlation existed between DACT2 expression levels and fibrosis severity (Table 4, Spearman rho=-0.476, P=0.010). These results suggest that DACT2 may inhibit fibrosis development in the myocardium.

Full table
Effects of DACT2 on AF related genes and the β-catenin pathway in the atrial myocyte line
DACT2 expression was rarely detected in the murine atrial muscle cell line HL-1, and it was overexpressed after infection with the adenovirus (Figure 2A,B). Compared with the results for the control group, DACT2 expression significantly increased the expression levels of KCNE5 (control vs. DACT2: P=0.002) and decreased the levels of KCNH2 (control vs. DACT2: P=0.009) and SCN5A (control vs. DACT2: P=0.001) but did not have any effect on KCNE1, KCNE2, KCNE4, KCNJ5, KCNQ1, CACNA1C or CACNB2 (Figure 2C,D,E,F,G,H,I,J,K,L). These results suggested that DACT2 could regulate electrical atrial remodeling in AF.
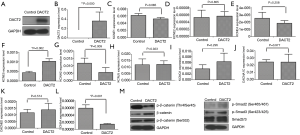
To deepen our understanding of the mechanism of DACT2 in AF, the activities of the Wnt and TGF-β signaling pathways were detected. DACT2 significantly decreased the level of p-β-catenin (Thr41/Ser45) while increasing the level of β-catenin, and had no obvious effect on the expression of p-β-catenin (Ser552), p-Smad2 (Ser465/467), p-Smad3 (Ser423/425) and Smad2/3 (Figure 2M). Phosphorylation at Thr41/Ser45 destabilizes β-catenin, while Ser552 phosphorylation induces β-catenin accumulation in the nucleus and increases its transcriptional activity (20-23). Our data showed that DACT2 could decrease β-catenin degradation and increase total β-catenin but it did not alter its transcriptional activity, suggesting that a new mechanism involved in the regulation by DACT2 on β-catenin in murine atrial muscle cells exists by which AF development is regulated.
DACT2 inhibited collagen I and collagen III expression and the TGF-β pathway in primary rat atrial fibroblasts
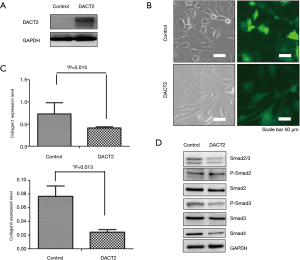
DACT2 was overexpressed in primary rat atrial fibroblasts (Figure 3A,B), and significantly decreased expression levels of collagen I and collagen III were detected by RT-PCR (Figure 3C). Further mechanistic analysis showed that DACT2 downregulated Smad2/3, Smad2, Smad3, p-Smad3 and Smad4 (Figure 3D), suggesting that DACT2 could inhibit the TGF-β pathway in primary rat atrial fibroblasts.
Discussion
DACT2 has been found to play important roles in embryonic development (7,8) and plays a protective role in hepatocellular carcinoma, esophageal squamous cell carcinoma, colon cancer, nasopharyngeal carcinoma and gastric cancer (5,6,13,14,24). However, little is known about its role in cardiac disease. Our data showed that decreased DACT2 expression levels were associated with AF and were correlated with fibrosis severity in patients with valvular heart disease (Figure 1, Tables 2 and 4), suggesting that DACT2 serves as a protective factor by regulating structural and electrical atrial remodeling in AF. To the best of our knowledge, this is the first report about the involvement of DACT2 in AF.
AF development is a complex process, in which multiple ionic channels and interstitial fibrosis are involved (25,26). In this study, DACT2 expression regulated the levels of potassium channel proteins (downregulation of KCNH2 and upregulation of KCNE5) and sodium ion channel proteins (downregulation of SCN5A) but not L-type calcium current channel proteins (CACNA1C and CACNB2) in HL-1 cells (Figure 2). Gain-of-function mutations in KCNE1–5 and loss-of function mutations in KCNH2 were associated with AF, while both gain- or loss-of-function-induced alterations in cardiac sodium current are involved in early-onset AF (27). The effect of DACT2 on the genes mentioned above suggested that DACT2 could contribute to the atrial action potential and intercellular conduction in AF. Furthermore, DACT2 inhibited collagen I and collagen III expression and the TGF-β pathway in primary rat atrial fibroblasts (Figure 3). TGFβ1 plays a critical role in the development of atrial fibrosis by promoting fibroblast proliferation and differentiation into collagen-secreting myofibroblasts (28). These results suggested that DACT2 could inhibit fibrosis formation by downregulating the TGF-β pathway. Taken together, the results suggest that DACT2 can regulate structural and electrical atrial remodeling in AF.
DACT2 has been reported to function not only as an antagonist of TGF-β/Nodal signaling (6-8), but also as an epigenetic regulator of Wnt signaling (12-14). Both of these signaling pathways play crucial roles in cardiac disease. TGF-β1 is associated with the selective development of fibrosis within the atria rather than the ventricles, and individuals in whom its expression levels are increased are prone to developing AF as a result of increased levels of atrial fibrosis (29,30). Wnt signaling activation results in myocardial hypertrophy, and is associated with left ventricular dilatation and reduction of EFs (31). DACT2 could affect β-catenin accumulation by reducing its phosphorylation at Thr41/Ser45 but not by affecting the TGF-β signaling pathway in atrial muscle cells, while it could inhibit the TGF-β signaling pathway in atrial fibroblasts (Figures 2N,3D), suggesting that the cellular specificity of the regulation mechanism might play a role in AF.
Conclusions
In summary, DACT2 downregulation was associated with AF and was correlated with fibrosis severity in patients with valvular heart disease. DACT2 can regulate electrical and structural remodeling by modulating the expression of potassium channel proteins and sodium ion channel proteins in atrial muscle cells and collagen protein in atrial fibroblasts. β-catenin accumulation in atrial muscle cells and TGF-β inhibition in atrial fibroblasts resulted from DACT2 expression. DACT2 may be a protective factor against AF in valvular heart disease.
This study is limited in that we could only obtain biopsies from the right atrial appendage. There could be heterogeneity in DACT2 expression in different parts of the heart but we were not able to obtain tissues from the left atria for examination. Another limitation is that the results of electrophysiological studies in HL-1 cells were different in terms of the determination of a phenotype according to changes in DACT2 expression.
Acknowledgments
We are grateful to Yuanjun Guan and Juan Li of the Core Lab for Medical Science, Zhongshan School of Medicine, Sun Yat-Sen University for technical assistance.
Funding: This research was supported by the National Key R&D Program of China (2017YFC1105000) and the National Natural Science Foundation of China (81900294, 81770319 and 81570039).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-4206). All authors report grants from National Key R&D Program of China, grants from National Natural Science Foundation of China, during the conduct of the study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee for clinical research and animal trials of the First Affiliated Hospital of Sun Yat-sen University ([2017]157). Informed consent was obtained before participation in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Murphy NF, Simpson CR, Jhund PS, et al. A national survey of the prevalence, incidence, primary care burden and treatment of atrial fibrillation in Scotland. Heart 2007;93:606-12. [Crossref] [PubMed]
- Mahida S, Lubitz SA, Rienstra M, et al. Monogenic atrial fibrillation as pathophysiological paradigms. Cardiovasc Res 2011;89:692-700. [Crossref] [PubMed]
- Kostin S, Klein G, Szalay Z, et al. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res 2002;54:361-79. [Crossref] [PubMed]
- Wijffels MC, Kirchhof CJ, Dorland R, et al. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995;92:1954-68. [Crossref] [PubMed]
- Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002;54:230-46. [Crossref] [PubMed]
- Hou J, Liao LD, Xie YM, et al. DACT2 is a candidate tumor suppressor and prognostic marker in esophageal squamous cell carcinoma. Cancer Prev Res (Phila) 2013;6:791-800. [Crossref] [PubMed]
- Su Y, Zhang L, Gao X, et al. The evolutionally conserved activity of Dapper2 in antagonizing TGF-beta signaling. FASEB J 2007;21:682-90. [Crossref] [PubMed]
- Zhang L, Zhou H, Su Y, et al. Zebrafish Dpr2 inhibits mesoderm induction by promoting degradation of nodal receptors. Science 2004;306:114-7. [Crossref] [PubMed]
- Alvarez D, Cardenes N, Sellares J, et al. IPF lung fibroblasts have a senescent phenotype. Am J Physiol Lung Cell Mol Physiol 2017;313:L1164-73. [Crossref] [PubMed]
- Sensiate LA, Sobreira DR, Da Veiga FC, et al. Dact gene expression profiles suggest a role for this gene family in integrating Wnt and TGF-beta signaling pathways during chicken limb development. Dev Dyn 2014;243:428-39. [Crossref] [PubMed]
- Schubert FR, Sobreira DR, Janousek RG, et al. Dact genes are chordate specific regulators at the intersection of Wnt and Tgf-β signaling pathways. BMC Evol Biol 2014;14:157. [Crossref] [PubMed]
- Wang S, Dong Y, Zhang Y, et al. DACT2 is a functional tumor suppressor through inhibiting Wnt/beta-catenin pathway and associated with poor survival in colon cancer. Oncogene 2015;34:2575-85. [Crossref] [PubMed]
- Yu Y, Yan W, Liu X, et al. DACT2 is frequently methylated in human gastric cancer and methylation of DACT2 activated Wnt signaling. Am J Cancer Res 2014;4:710-24. [PubMed]
- Zhang X, Yang Y, Liu X, et al. Epigenetic regulation of the Wnt signaling inhibitor DACT2 in human hepatocellular carcinoma. Epigenetics 2013;8:373-82. [Crossref] [PubMed]
- Hou J, Yue Y, Hu B, et al. DACT1 Involvement in the Cytoskeletal Arrangement of Cardiomyocytes in Atrial Fibrillation by Regulating Cx43. Braz J Cardiovasc Surg 2020;34:711-22. [PubMed]
- Kirchhof P, Kahr PC, Kaese S, et al. PITX2c is expressed in the adult left atrium, and reducing Pitx2c expression promotes atrial fibrillation inducibility and complex changes in gene expression. Circ Cardiovasc Genet 2011;4:123-33. [Crossref] [PubMed]
- Wang LX, Yang X, Yue Y, et al. Imatinib attenuates cardiac fibrosis by inhibiting platelet-derived growth factor receptors activation in isoproterenol induced model. PLoS One 2017;12:e0178619. [Crossref] [PubMed]
- Li D, Fareh S, Leung TK, et al. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation 1999;100:87-95. [Crossref] [PubMed]
- Levy S. Factors predisposing to the development of atrial fibrillation. Pacing Clin Electrophysiol 1997;20:2670-4. [Crossref] [PubMed]
- Amit S, Hatzubai A, Birman Y, et al. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev 2002;16:1066-76. [Crossref] [PubMed]
- Liu C, Li Y, Semenov M, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002;108:837-47. [Crossref] [PubMed]
- Yanagawa S, Matsuda Y, Lee JS, et al. Casein kinase I phosphorylates the Armadillo protein and induces its degradation in Drosophila. EMBO J 2002;21:1733-42. [Crossref] [PubMed]
- Fang D, Hawke D, Zheng Y, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem 2007;282:11221-9. [Crossref] [PubMed]
- Li L, Zhang Y, Fan Y, et al. Characterization of the nasopharyngeal carcinoma methylome identifies aberrant disruption of key signaling pathways and methylated tumor suppressor genes. Epigenomics 2015;7:155-73. [Crossref] [PubMed]
- Fatkin D, Otway R, Vandenberg JI. Genes and atrial fibrillation: a new look at an old problem. Circulation 2007;116:782-92. [Crossref] [PubMed]
- Jalife J, Kaur K. Atrial remodeling, fibrosis, and atrial fibrillation. Trends Cardiovasc Med 2015;25:475-84. [Crossref] [PubMed]
- Olesen MS, Nielsen MW, Haunso S, et al. Atrial fibrillation: the role of common and rare genetic variants. European Journal of Human Genetics 2014;22:297-306. [Crossref] [PubMed]
- Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 2008;1:62-73. [Crossref] [PubMed]
- Nakajima H, Nakajima HO, Salcher O, et al. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res 2000;86:571-9. [Crossref] [PubMed]
- Verheule S, Sato T. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res 2004;94:1458-65. [Crossref] [PubMed]
- Malekar P, Hagenmueller M, Anyanwu A, et al. Wnt signaling is critical for maladaptive cardiac hypertrophy and accelerates myocardial remodeling. Hypertension 2010;55:939-45. [Crossref] [PubMed]


