
Prognostic impact of lymphadenectomy on outcomes of sublobar resection for non-small cell lung cancer ≤1 or >1 to 2 cm
Introduction
Lung cancer is the leading cause of cancer-related deaths, and non-small cell lung cancer (NSCLC) accounts for nearly 85% of lung cancer (1). The 2020 National Comprehensive Cancer Network (NCCN) guidelines recommend lobectomy combined with systematic mediastinal lymph node (LN) dissection as the standard surgical procedure for NSCLC, which identifies patients who may benefit from subsequent chemotherapy and target therapy (2). However, in patients without nodal metastases, prophylactic lymphadenectomy has limited benefit for survival (3). Moreover, mediastinal lymphadenectomy may increase the drainage and operative time, and may cause damage to neurogenic, vascular, and lymphatic structures in the mediastinum (4).
With the widespread use of high-resolution computed tomography (HRCT), more and more small-sized (≤2 cm) NSCLC are being detected (5). Sublobar resection as an alternative choice for small-sized (≤2 cm) NSCLC can achieve a similar prognosis to that of lobectomy, and its use is increasing (6-10). However, the optimal number of LNs to be dissected for patients with NSCLC 2 cm or less during sublobar resection has not been standardized (11-13). Moreover, the 8th tumor, node, and metastases (TNM) classification system subclassified NSCLC tumors 2 cm or less into T1a (≤1 cm) and T1b (>1 to 2 cm) disease owing to the significantly better prognosis for patients with NSCLC ≤1 cm (14). Furthermore, patients with NSCLC ≤1 cm have a lower probability of mediastinal LN metastasis than those with tumors >1 to 2 cm (15,16). However, it is still not clear whether patients with NSCLC ≤1 cm could benefit from less extensive LN dissection.
Hence, this study aimed to investigate the appropriate extent of LN dissection for patients with NSCLC ≤1 cm and >1 to 2 cm after sublobar resection, based on the Surveillance, Epidemiology, and End Results (SEER) database.
Methods
Study population
All the patients in our study were selected from the SEER 21 database which covered 21 geographically different registries and made up of 34.6% of the United States population (17). Patients who met the following inclusion criteria were included: (I) pathologically diagnosed as primary NSCLC between January 2010 and November 2015, (II) tumor size ≤2 cm, (III) and wedge resection or segmentectomy performed with or without lymphadenectomy. The exclusion criteria were as follows: (I) insufficient information on the number of resected LNs and clinicopathological characteristics, (II) with another malignant primary tumor during follow-up period and (III) received chemotherapy and/or radiotherapy before the surgery.
Data collection
Demographic variables included age, sex, race and marital status were obtained. Tumor characteristics were consisted of tumor location, histological type, tumor size, and grade. Treatment information including surgical procedures and number of dissected LNs were collected from the SEER database. All the patients were divided into three groups based on the extent of LNs dissection: no-LN dissection, 1 to 3 LNs dissection and ≥4 LNs dissection. Overall survival (OS) was defined as the time from surgery until death from any cause. Lung cancer-specific survival (LCSS) was defined as the time from surgery until death because of lung cancer. The survival time and the cause of death whether due to lung cancer were retrieved from the specific codes provided by SEER database. The last follow-up date was January 2016.
Statistical analysis
All the data were either shown as mean ± standard deviation or number (percent values). We used Pearson χ2 test to compare categorical variables and one-way analysis of variance (ANOVA) to compare the continuous variables between different groups. Log-rank test and Cox proportional-hazards regression model were applied to evaluate predictive factors of OS and LCSS. In the current study, a two-sided P value of less than 0.05 was considered statistically significant. All analyses were performed using SPSS 26.0 (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
Overall, 7,627 patients with NSCLC ≤2 cm who underwent sublobar resection were included. The median follow-up time was 33 months. Among them, 3,424 (44.9%) patients underwent no-LN dissection, 1,957 (25.7%) patients underwent 1 to 3 regional LNs dissection and 2,246 (29.4%) patients underwent ≥4 regional LNs dissection. The detailed baseline information of patients was shown in Table 1. Patients with ≥4 LNs dissection and 1 to 3 LNs dissection were more likely to have lager tumor size (P<0.001), greater proportion of adenocarcinoma (P<0.001) and undergoing segmentectomy (P<0.001) compared those with no-LN dissection. No significant differences were found on age, sex, race, marital status, and tumor grade among these groups. We subsequently divided patients into T1a (≤1 cm, n=2,041, 26.8%) and T1b (>1 to 2 cm, n=5,586, 73.2%) subgroups, and the baseline characteristics were shown in Table S1.
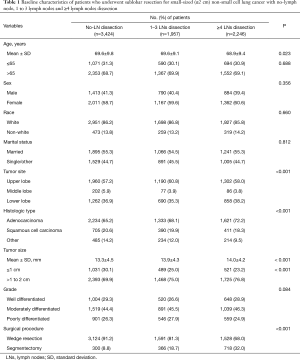
Full table
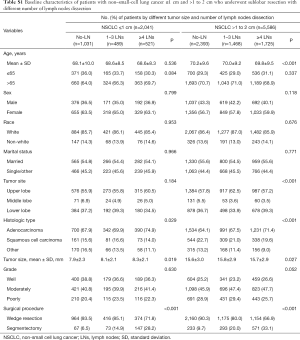
Full table
Prognostic impact of lymphadenectomy on outcomes for NSCLC ≤2 cm after sublobar resection
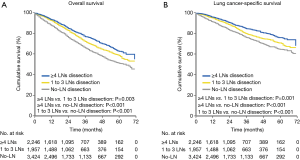
The extent of LNs dissection was associated with the OS and LCSS in patients with NSCLC ≥2 cm after sublobar resection. The 5-year OS rates were 62.5%, 58.3% and 50.9% for patients with ≥4 LNs dissection, 1 to 3 LNs dissection and no-LN dissection, respectively. The 5-year LCSS rates were 75.8%, 70.2% and 64.5% for patients with ≥4 LNs dissection, 1 to 3 LNs dissection and no-LN dissection, respectively. The log-rank test indicated that patients with ≥4 LNs dissection had significantly better survival compared with those who had 1 to 3 LNs dissection and no-LN dissection in OS (≥4 LNs dissection versus 1 to 3 LNs dissection: P=0.003; ≥4 LNs dissection versus no-LN dissection: P<0.001) and LCSS (≥4 LNs dissection versus 1 to 3 LNs dissection: P=0.001; ≥4 LNs dissection versus no-LN dissection: P<0.001). In addition, the survival analysis also indicated better prognosis of patients after 1 to 3 LNs dissection compared those with no-LN dissection (OS, P<0.001; LCSS, P<0.001) (Figure 1A,B).
Further multivariate Cox proportional-hazards regression model demonstrated that patients with 1 to 3 LNs dissection [OS: hazard ratio (HR), 1.169; 95% confidence interval (CI), 1.025–1.332; P=0.020; LCSS: HR, 1.268; 95% CI, 1.075–1.495; P=0.005] and no-LN dissection (OS: HR, 1.529; 95% CI, 1.363–1.716; P<0.001; LCSS: HR, 1.669; 95% CI, 1.442–1.931; P<0.001) were independent predictors of poorer survival than those with ≥4 LNs dissection. Moreover, older age (OS: HR, 1.239; 95% CI, 1.120–1.370; P<0.001; LCSS: HR, 1.134; 95% CI, 1.003–1.283; P=0.044), non-married status (OS: HR, 1.179; 95% CI, 1.076–1.291; P<0.001), moderately differentiated (OS: HR, 1.692; 95% CI, 1.493–1.918; P<0.001; LCSS: HR, 1.930; 95% CI, 1.635–2.277; P<0.001) and poorly differentiated (OS: HR, 1.829; 95% CI, 1.660–2.014; P<0.001; LCSS: HR, 2.243; 95% CI, 1.986–2.533; P<0.001) were other independent predictors of worse survival (Table 2).
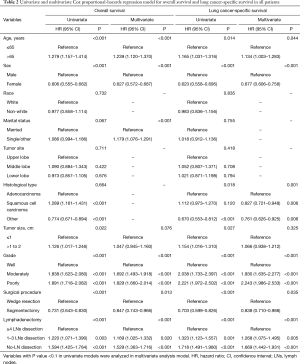
Full table
Prognostic impact of lymphadenectomy on outcomes for NSCLC ≤1 cm after sublobar resection
The log-rank test showed that patients with ≥4 LNs dissection and 1 to 3 LNs dissection had significantly better prognosis compared with patients who had no-LN dissection in OS (≥4 LNs dissection versus no-LN dissection: P<0.001; 1 to 3 LNs dissection versus no-LN dissection: P<0.001) and LCSS (≥4 LNs dissection versus no-LN dissection: P<0.001; 1 to 3 LNs dissection versus no-LN dissection: P=0.003). However, patients with ≥4 LNs dissection had equivalent OS (P=0.267) and LCSS (P=0.323) compared with those who had 1 to 3 LNs dissection (Figure 2A,B).
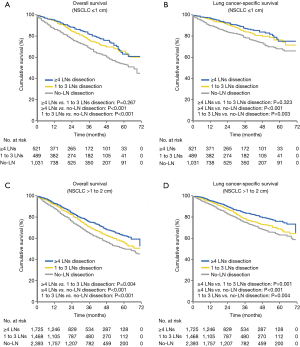
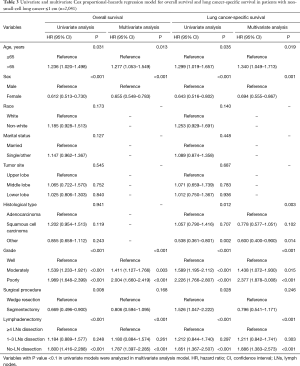
Subsequently multivariate Cox proportional-hazards regression model revealed that no-LN dissection was an independent risk factor of worse OS (HR, 1.787; 95% CI, 1.397–2.285; P<0.001) and LCSS (HR, 1.886; 95% CI, 1.383–2.573; P<0.001), whereas 1 to 3 LNs dissection was not an independent risk factor of worse survival compared with ≥4 LNs dissection (OS: HR, 1.180; 95% CI, 0.884–1.574; P=0.261, LCSS: HR, 1.211; 95% CI, 0.842–1.741; P=0.303) (Table 3).
Full table
Prognostic impact of lymphadenectomy on outcomes for NSCLC >1 to 2 cm after sublobar resection
The log-rank test showed that patients with ≥4 LNs dissection had significantly better prognosis compared with those who had 1 to 3 LNs and no-LN dissection in both OS (≥4 LNs dissection versus 1 to 3 LNs dissection: P=0.004; ≥4 LNs dissection versus no-LN dissection: P<0.001) and LCSS (≥4 LNs dissection versus 1 to 3 LNs dissection: P=0.001; ≥4 LNs dissection versus no-LN dissection: P<0.001). Moreover, patients with 1 to 3 LNs dissection also achieved better survival than those with no-LN dissection in both OS (1 to 3 LNs dissection versus no-LN dissection: P<0.001) and LCSS (1 to 3 LNs dissection versus no-LN dissection: P=0.004) (Figure 2C,D).
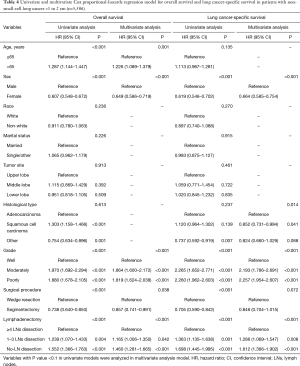
Multivariate Cox proportional-hazards regression model indicated that patients with no-LN dissection (OS: HR, 1.460, 95% CI, 1.281–1.665, P<0.001; LCSS: HR, 1.612, 95% CI, 1.366–1.902, P<0.001) and 1 to 3 LNs dissection (OS: HR, 1.165, 95% CI, 1.006–1.350, P=0.042; LCSS: HR, 1.286, 95% CI, 1.069–1.547, P=0.008) were independent risk factors of worse survival compared those with ≥4 LNs dissection (Table 4).
Full table
Discussion
Lymphadenectomy is an important part of the surgical treatment for early-stage NSCLC and can indicate which patients would benefit from subsequent adjuvant therapy. However, the prognostic impact of LN dissection for patients with NSCLC ≤1 cm and >1 to 2 cm who underwent sublobar resection is still unclear. This study indicated that lymphadenectomy has an important impact on NSCLC ≤2 cm after sublobar resection. For NSCLC >1 to 2 cm, the removal of at least 4 LNs was recommended. More importantly, for patients with NSCLC ≤1 cm, the dissection of 1 to 3 LNs achieved a similar survival compared those with dissection of ≥4 LNs. Our findings have important practical implications for lymphadenectomy for patients with NSCLC ≤2 cm after sublobar resection.
Lymphadenectomy is used for accurate node staging, the detection of occult node metastases, and the guidance of postoperative adjuvant chemotherapy or radiotherapy. It has been demonstrated that incomplete LN retrieval would seriously affect the accurate nodal classification (18,19), but complete mediastinal LN dissection may increase the operative time and morbidity and chest tube drainage (4). Previous study found that there was no association between the extent of lymphadenectomy and the survival benefit for patients with early-stage NSCLC, but they mainly focused on lobectomy (20). By now, the prognostic impact of lymphadenectomy on survival outcomes for patients who undergo sublobar resection is rarely reported. Hence, it is vital to determine the optimal number of LN that should be resected to achieve a balance between the adequate evaluation of the nodal status and minimizing surgical trauma.
Surgical resection is the most effective treatment strategy for early-stage NSCLC according to the 2020 NCCN guideline (2). Previous studies have strongly demonstrated that sublobar resection can achieve equivalent long-term survival compared with lobectomy for early-stage NSCLC (21-24). Despite the ongoing controversies about the adequacy of sublobar resection for treatment (25,26), its use in early-stage NSCLC is increasing. Clinically, sublobar resection is generally considered acceptable for peripheral small-sized (≤2 cm) NSCLC (9,10). Compared with the standard surgical procedure of lobectomy plus systematic mediastinal lymphadenectomy (27), the significance of lymphadenectomy in sublobar resection is still unclear. The proportion of patients who underwent sublobar resection without lymphadenectomy is significantly higher than lobectomy (28,29). Our study also included a high proportion (44.9%) of patients who did not undergo lymphadenectomy after sublobar resection. Moreover, Patients with NSCLC ≤2 cm who underwent sublobar resection plus dissection of ≥4 LNs or 1 to 3 LNs had better survival outcomes than patients who did not have LN dissection. These results indicated the importance of lymphadenectomy in NSCLC patients who underwent sublobar resection.
Several studies have discussed the association between lymphadenectomy and survival outcomes in patients who underwent sublobar resection for early-stage NSCLC (3,11,13,29). Yendamuri et al. demonstrated that the examination of nine or more LNs was associated with improved survival in patients with stage IA NSCLC (≤2 cm) after sublobar resection due to the avoidance of inaccurate staging and guidance of adjuvant therapy postoperatively (12). Recently, Cao et al. revealed that ≥4 LNs dissection achieved superior survival compared with dissection of 1 to 3 LNs and no-LN dissection in OS and LCSS for patients with NSCLC 2 cm or less (11), this was consistent with our results. However, Liu et al. found that ≥7 resected LNs was associated with better prognosis for stage IA NSCLC undergoing sublobar resection (13). The ongoing randomized controlled trials will address the controversial issues of appropriate LNs dissection and provide high-quality evidence in the future (30,31).
The 8th TNM classification subclassified tumors ≤2 cm as T1a (≤1 cm) and T1b (>1 to 2 cm) disease because of the different prognoses of the two subgroups (14,32). Patients with NSCLC ≤1 cm have lower risks of LN metastasis and recurrence than patients with tumors >1 to 2 cm (15,33). However, the impact of lymphadenectomy on the prognosis for T1a and T1b disease remains unclear. Ding et al. found that the survival benefit peaked with the resection of 4 to 9 LNs in NSCLC ≤1 cm and ≥10 LNs in NSCLC >1 to 2 cm for patients after wedge resection based on the National Cancer Database (NCDB) cohort (3). However, our study showed that dissection of ≥4 LNs or 1 to 3 LNs had similar survival outcomes in NSCLC ≤1 cm. As to NSCLC >1 to 2 cm, dissection of ≥4 LNs achieved a better survival than dissection of 1 to 3 LNs for patients who underwent sublobar resection. These results indicate that sufficient lymphadenectomy remains an important part of surgical resection and surgeons should attach more importance to lymphadenectomy in sublobar resection even for small-sized NSCLC.
The tumor size is an important component of surgical decision making and prognostic evaluation. Chen et al. reported that NSCLC >1 to 2 cm had a higher rate of nodal metastasis than NSCLC 1 cm or less (13.8% versus 3.5%) (15). These results indicated that small-sized NSCLC patients are a heterogeneous group, some of whom are potentially candidates for less extensive lymphadenectomy. In this study, we found that the dissection of ≥4 LNs does not improve the survival than dissection of 1 to 3 LNs in patients with NSCLC ≤1 cm undergoing sublobar resection. This finding has significant implications for surgical decisions in reducing surgical trauma.
We must acknowledge some limitations in this study. First, because of the nature of this retrospective study, performance bias and selection bias were inevitable. For example, the patients included in the presented study were not designed for lymphadenectomy after sublobar resection. Future randomized trials are necessary to validate our findings. Second, the respective number of dissected N1 and N2 LNs were not recorded in the SEER database, hence it is impossible to determine the appropriate number of LNs dissection specific to different location in sublobar resection. Third, the presence of ground-glass opacity (GGO) component on CT scan is associated with better survival outcomes (34,35) and 8th TNM classification also recommended the invasive component size, rather than the whole tumor size, is considered a better measure for T staging (14). However, the information on consolidation diameter and presence or absence of GGO component are not available in SEER database. Fourth, we evaluated the impact of LNs dissection on prognosis in sublobar resection but not specific to segmentectomy and wedge resection subgroups.
Conclusions
The extent of LNs dissection is associated with the survival in patients with NSCLC ≤2 cm after sublobar resection. Dissection of ≥4 LNs has superior survival compared with 1 to 3 LNs dissection in NSCLC >1 to 2 cm, whereas has equivalent survival for NSCLC ≤1 cm. Hence, dissection of ≥4 LNs should be recommended for NSCLC >1 to 2 cm, whereas surgeons could rely on surgical skills and the patient profile to decide ≥4 LNs or 1 to 3 LNs dissection for NSCLC ≤1 cm after sublobar resection.
Acknowledgments
We acknowledge the efforts of the SEER program tumor registries in the creation of the SEER database.
Funding: This study is supported by Ningbo Science and Technology Bureau (2016C50032) and Ningbo Health Branding Subject Fund (Grant No. PPXK2018-05).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/jtd-19-3773). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approval was waived by the local ethics committee, as SEER data is publicly available and deidentified.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines): non-small cell lung cancer, version 1. 2020. Available online: https://www.nccn.org/patients/guidelines/cancers.aspx#nsclc
- Ding H, Wang H, Xu L, et al. Survival and Resected Lymph Node Number During Sublobar Resection for N0 Non-Small Cell Lung Cancer 2 cm or Less. Ann Thorac Surg 2019;107:1647-55. [Crossref] [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [Crossref] [PubMed]
- National Lung Screening Trial Research T, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Kodama K, Higashiyama M, Okami J, et al. Oncologic Outcomes of Segmentectomy Versus Lobectomy for Clinical T1a N0 M0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:504-11. [Crossref] [PubMed]
- Moon Y, Lee KY, Park JK. Prognosis After Sublobar Resection of Small-sized Non-small Cell Lung Cancer with Visceral Pleural or Lymphovascular Invasion. World J Surg 2017;41:2769-77. [Crossref] [PubMed]
- Winckelmans T, Decaluwé H, De Leyn P, et al. Segmentectomy or lobectomy for early-stage non-small-cell lung cancer: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Zhong C, Sakurai H, Wei S, et al. Sublobar resections for small-sized stage Ia lung adenocarcinoma: a Sino-Japanese multicenter study. J Thorac Dis 2018;10:991-8. [Crossref] [PubMed]
- Koike T, Koike T, Sato S, et al. Lobectomy and limited resection in small-sized peripheral non-small cell lung cancer. J Thorac Dis 2016;8:3265-74. [Crossref] [PubMed]
- Cao J, Xu J, He Z, et al. Prognostic impact of lymphadenectomy on outcomes of sublobar resection for stage IA non-small cell lung cancer ≤2 cm. J Thorac Cardiovasc Surg 2018;156:796-805.e4. [Crossref] [PubMed]
- Yendamuri S, Dhillon SS, Groman A, et al. Effect of the number of lymph nodes examined on the survival of patients with stage I non-small cell lung cancer who undergo sublobar resection. J Thorac Cardiovasc Surg 2018;156:394-402. [Crossref] [PubMed]
- Liu Y, Shen J, Liu L, et al. Impact of examined lymph node counts on survival of patients with stage IA non-small cell lung cancer undergoing sublobar resection. J Thorac Dis 2018;10:6569-77. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Chen B, Wang X, Yu X, et al. Lymph node metastasis in Chinese patients with clinical T1 non-small cell lung cancer: A multicenter real-world observational study. Thorac Cancer 2019;10:533-42. [Crossref] [PubMed]
- Yu X, Li Y, Shi C, et al. Risk factors of lymph node metastasis in patients with non-small cell lung cancer ≤ 2 cm in size: A monocentric population-based analysis. Thorac Cancer 2018;9:3-9. [Crossref] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) program. Public-use data (1975-2016). Available online: https://seer.cancer.gov/
- Liang W, He J, Shen Y, et al. Impact of Examined Lymph Node Count on Precise Staging and Long-Term Survival of Resected Non-Small-Cell Lung Cancer: A Population Study of the US SEER Database and a Chinese Multi-Institutional Registry. J Clin Oncol 2017;35:1162-70. [Crossref] [PubMed]
- Ramirez RA, Wang CG, Miller LE, et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J Clin Oncol 2012;30:2823-8. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Taioli E, Yip R, Olkin I, et al. Survival after Sublobar Resection for Early-Stage Lung Cancer: Methodological Obstacles in Comparing the Efficacy to Lobectomy. J Thorac Oncol 2016;11:400-6. [Crossref] [PubMed]
- Chiang XH, Hsu HH, Hsieh MS, et al. Propensity-Matched Analysis Comparing Survival After Sublobar Resection and Lobectomy for cT1N0 Lung Adenocarcinoma. Ann Surg Oncol 2020;27:703-15. [Crossref] [PubMed]
- Okami J, Ito Y, Higashiyama M, et al. Sublobar resection provides an equivalent survival after lobectomy in elderly patients with early lung cancer. Ann Thorac Surg 2010;90:1651-6. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Veluswamy RR, Ezer N, Mhango G, et al. Limited Resection Versus Lobectomy for Older Patients With Early-Stage Lung Cancer: Impact of Histology. J Clin Oncol 2015;33:3447-53. [Crossref] [PubMed]
- Zhang J, Mao T, Gu Z, et al. Comparison of complete and minimal mediastinal lymph node dissection for non-small cell lung cancer: Results of a prospective randomized trial. Thorac Cancer 2013;4:416-21. [Crossref] [PubMed]
- Mohiuddin K, Haneuse S, Sofer T, et al. Relationship between margin distance and local recurrence among patients undergoing wedge resection for small (≤2 cm) non-small cell lung cancer. J Thorac Cardiovasc Surg 2014;147:1169-75; discussion 1175-7. [Crossref] [PubMed]
- Stiles BM, Mao J, Harrison S, et al. Extent of lymphadenectomy is associated with oncological efficacy of sublobar resection for lung cancer ≤2 cm. J Thorac Cardiovasc Surg 2019;157:2454-2465.e1. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Comparison of different types of surgery in treating patients with stage IA non379 small cell lung cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00499330
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Lee PC, Korst RJ, Port JL, et al. Long-term survival and recurrence in patients with resected non-small cell lung cancer 1 cm or less in size. J Thorac Cardiovasc Surg 2006;132:1382-9. [Crossref] [PubMed]
- Su H, Dai C, Xie H, et al. Risk Factors of Recurrence in Patients With Clinical Stage IA Adenocarcinoma Presented as Ground-Glass Nodule. Clin Lung Cancer 2018;19:e609-17. [Crossref] [PubMed]
- Mao R, She Y, Zhu E, et al. A Proposal for Restaging of Invasive Lung Adenocarcinoma Manifesting as Pure Ground Glass Opacity. Ann Thorac Surg 2019;107:1523-31. [Crossref] [PubMed]


