
Real-life effectiveness of fluticasone furoate/vilanterol after switching from fluticasone/salmeterol or budesonide/formoterol therapy in patients with symptomatic asthma: Relvar Ellipta for Real Asthma Control Study (RERACS study)
Introduction
The goals of asthma management are to achieve good control of symptoms and maintain normal activity. In spite of effective therapies being available, international surveys show that asthma control is suboptimal in many countries (1). Although the situation varies between countries, it is problematic that from 50% to 90% of asthma patients show poor control of their disease (2-5). There are various reasons for poor control of asthma. Common causes related to medication are poor adherence, poor inhaler technique, and inadequate dosing. If possible, potential risk factors should be eliminated and comorbidities managed (1,6,7).
New users of both single and fixed combined inhaled corticosteroids (ICS) have very low persistence rates with ICS treatment during the first year of follow-up and persistence with treatment is strongly influenced by patient factors, such as the severity of asthma and the daily dosing frequency (8). Good adherence is associated with fewer exacerbations, but the difference is only significant for patients whose adherence is greater than 75% of the prescribed dose compared with patients whose adherence is 25% or less (9). In a real-world study, not a clinical trial, adherence was higher among patients prescribed once-daily ICS compared with those prescribed ICS ≥2 times daily as, 61% vs. 41%, respectively (10). This trend was similar regardless of sex, ethnicity, age, and the severity of asthma. A study of step-down therapy also showed that adherence was higher among asthma patients prescribed once daily ICS compared with those prescribed ICS 2 times daily (76.0% vs. 58.7%, respectively), and clinical parameters also showed greater improvement in patients using once-daily ICS (11).
The Salford Lung Study evaluated the effectiveness and safety of switching to the once-daily inhaled combination of fluticasone furoate and vilanterol (FF/VI, Relvar®Elipta®) compared with continuation of maintenance therapy (usual care) in a large, real-world population of patients with chronic obstructive pulmonary disease (COPD) and asthma (12). In patients with a diagnosis of symptomatic asthma made by a general practitioner on maintenance inhaler therapy, including single ICS and ICS/long-acting beta-agonist (ICS/LABA), initiation of a once-daily FF/VI regimen improved asthma control without increasing the risk of serious adverse events compared with optimized usual care (13). Subgroup analysis also showed that initiating FF/VI was significantly better than continuing fluticasone propionate/salmeterol (FP/SM) for improving asthma control and quality of life (14). These reports suggest that once-daily treatment improves asthma control, but the efficacy of switching from FP/SM or budesonide/formoterol (BD/FM) to FF/VI at the equivalent corticosteroid dose and the changes of biomarkers has not been tested in a real-world study. Therefore, the objective of this study, the Relvar Ellipta Real Asthma Control Study (RERACS study), was to evaluate the efficacy of switching therapy from FP/SM or BD/FM to FF/VI at the equivalent corticosteroid dose with measuring biomarkers in the real-world setting.
Methods
Study design
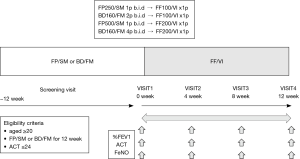
A prospective, 3-month, open-label, parallel group, switching therapy trial was performed in symptomatic asthma patients at Dokkyo University Hospital, Japan to assess the effectiveness of switching from FP/SM or BD/FM to FF/VI. Each patient’s ICS was switched to the equivalent dose according to the previously described method (1). Patients using 1 puff of FP 250 µg/SM 50 µg (FP250/SM) b.i.d or 2 puffs of BD 160 µg /FM 4.5 µg (BD160/FM) b.i.d were switched to FF 100 µg/VI 25 µg (FF100/VI) once daily, while patients using 1 puff of FP 500 µg/SM 50 µg (FP500/SM) b.i.d or 4 puffs of BD160/FM b.i.d were switched to FF 200 µg/VI 25 µg (FF200VI) once daily (Figure 1). The primary outcome was improvement of the predicted percent forced expiratory volume in 1 second (%FEV1). The measurement was performed at the time in the morning to noon, and the regular use of ICS/LABA was not stopped. Secondary outcomes were improvement of asthma symptoms evaluated by the asthma control test (ACT) and the change of fractional exhaled nitric oxide (FeNO). The ACT score was used to classify patients as follows: ACT <20 was poor control, ACT ≥20 and ≤24 was good control, and ACT =25 was complete control. %FEV1 and FeNO (Sievers Instruments, Boulder, CO, USA) were determined as described previously (15-17). The screening visit was at 12 weeks prior to switching therapy and patients were switched to FF/VI at Visit 1 (week 0). Parameters were measured every 4 weeks from visit 1 (week 0) to visit 4 (week 12). This study was designed to have 90% power to detect a 1% (20 mL) difference of switching therapy effect during 3 months in %FEV1 with effective size 0.74 and two-sided alpha of 0.05 (13,18). A sample size of 32 patients was planned. For secondary outcome of ACT and FeNO, a sample size of 1 point with effective size 0.75, and a sample size 10 ppb with effective size 0.8 were sufficient to construct 90% power to detect in each parameter (18,19).
Patients
Eligibility criteria included asthma patients aged ≥20 years, use of FP/SM or BD/FM for at least 3 months (12 weeks) prior to enrollment this study, symptomatic asthma (ACT ≤24), and informed consent to participation in the study. Exclusion criteria were an age <20 years, ACT =25, intercurrent infection, and known or suspected allergy to FF/VI. Asthma was managed according to the 2014 Japanese asthma treatment guideline (20). This study was conducted in accordance with the Declaration of Helsinki. All patients gave written informed consent before enrollment. The Human Research Committee of Dokkyo University approved this study and it was registered as clinical trial number C-274-03.
Safety
Safety endpoints included severe adverse events (SAEs) and the percentage of patients who stopped medication during the study period.
Statistical analysis
Results are expressed as the mean ± SD. Statistical analysis were carried out by one-way ANOVA followed by Bonferroni test and statistical significance compared to baseline was accepted at P<0.05.
Results
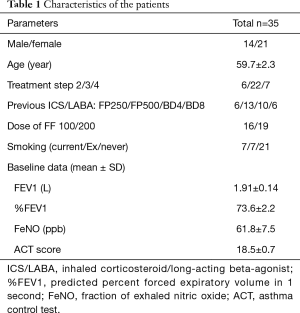
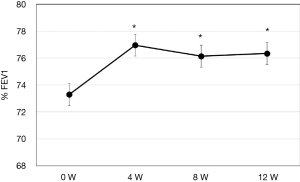
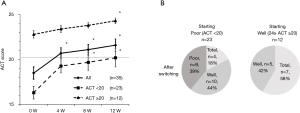
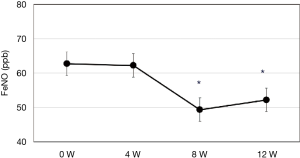
Clinical characteristics of the patients are shown in Table 1. A total of 35 patients were enrolled. They showed female predominance and most were in asthma treatment step 3. A similar number of patients were switched to FF100/VI or FF200/VI. The mean ACT score was 18.5±0.7, indicating insufficient control of asthma. The primary endpoint (%FEV1) showed improved at 4 weeks after switching therapy, and this improvement was maintained until 12 weeks (P<0.05) (Figure 2). ACT also improved after switching therapy (P<0.05). Patients with an ACT <20 displayed marked improvement of their symptoms at 4 weeks and 62% of them had a score >20. Patients with an ACT ≥20 also demonstrated improvement of asthma symptoms at 12 weeks and 58% of them reached ACT =25, indicating complete control (Figure 3A,B). FeNO was decreased at 8 weeks, with this level being maintained until 12 weeks (P<0.05) (Figure 4).
Full table
Safety assessment revealed that no patient stopped medication or developed pneumonia during the study period. Hoarseness was noted in three patients as an adverse event.
Discussion
In the present study, switching symptomatic asthma patients from FP/SM or BD/FM to FF/VI improved their asthma symptoms evaluated by the ACT score, %FEV1, and FeNO. These results indicated that switching therapy from FP/SM or BD/FM to FF/VI improved symptoms and lung function in symptomatic asthma patients, and also reduced airway inflammation, despite changing inhalation from twice daily to once daily at the equivalent ICS dose. There are several reasons why once daily treatment may have led to improvement. The first is that adherence may have improved. It may also be important that the inhaled steroid in FF/VI has a strong anti-inflammatory effect. A third factor is that the patients were switched to an easy-to-use device that makes erroneous operation unlikely. Accordingly, the effectiveness of switching to FF/VI may have been supported by all three factors. Adherence differs between clinical trials and the real-world setting. In a clinical study of asthma, patients must pass strict selection criteria to be registered. Thus, if there are initially 300 asthma patients and the %FEV1 criterion is set within 50–85%, the number of patients is reduced to 1/3. In addition, smokers are excluded, as well as patients with less than 12% airway reversibility in the past year, and so on. As a result, from the original 300 asthma patients, only 11 patients may be eligible for a clinical study (21). This is quite different from actually investigating the effect of a drug in routine clinical practice. In randomized controlled trials, adherence to inhaled drugs is more than 90% if patient diary cards used to assess drug use (22), while observational studies show very low adherence rates of less than 20% (8,23). Since there is a large gap between clinical trials and real-world medicine, there is a possibility that a large difference will arise when comparing twice daily inhalation and once daily inhalation (24). In a study performed during real-world clinical practice, it was clearly demonstrated that adherence was increased by once daily inhalation (11).
As for its anti-inflammatory effect, FF is a derivative of FP that has the highest affinity for glucocorticoid receptors among the existing inhaled steroids, followed by mometasone and budesonide (25). The LABA component of VI has a persistent adrenoceptor (β2-AR) agonist action comparable with that of indacaterol and longer than FM (26). Since FF100/VI provides an equivalent corticosteroid dose to FP250/SM, it is expected to achieve the same degree of asthma control from both its ICS and LABA components. In fact, once daily FF100/VI achieved similar improvement of %FEV1 compared with twice daily FP250/SM. However, FF100/VI was better for QOL, when comparing the proportion of patients with significant improvement of AQLQ by at least 0.5 (27). That study was a phase 3 randomized controlled trial, so it is considered that adherence was probably good, but a difference was still noted after switching therapy.
The difference in the duration of the anti-inflammatory effect might be important. It was reported that FeNO decreased 1 week after starting FF/VI, while FeNO increased to the level obtained with placebo at 18 days after discontinuation of FF/VI, indicating that the anti-inflammatory effect of FF/VI was sustained for 18 days (28). The duration of the anti-inflammatory effect of BD, FP, and beclomethasone estimated from FeNO was 7, 14, and 7–14 days, respectively (29-31). The bronchodilatory effect evaluated by FEV1 and peak expiratory flow was also sustained for 4 days longer after cessation of FF/VI (28). In present study, mean FeNO values were decreased by switching therapy, but still above 50 ppb, nevertheless improving ACT and %FEV1. Response to ICS could be different from high-FeNO >100 ppb patients and low-FeNO >60 to 100 (30). The high-FeNO patients showed progressive fall in FeNO according to ICS dosing up, but low-FeNO patients showed modest fall. The levels of FeNO seemed to be affected the discordance of between ACT score and objective measures such as %FEV1 and FeNO. Another reason to discordance might be poor adherence rates. The study for real-world revealed the poor adherence rate as low as 20–40% by examining electric monitoring devise (13).
Finally, as already mentioned, FF/VI is inhaled once a day, and 95% of patients can handle it successfully from the first use (32), which is also a very important point for an inhaled medication.
Limitations of this study are that monocentric, without blinding or control group and small population. Precise background of patients, such as blood eosinophil count, airway reversibility and chest computed tomography were also not examined. The purpose of this study was to reveal asthma control in real world. Therefore, to avoid the controlled adherence based on clinical study, adherence was also not examined by questionnaires or automatic recorders. It might be possible to track adherence by examining Elipta® device turned in at the end of the study. A recent report referencing the Salford Lung Study suggested the key learnings for the design of future pragmatic effectiveness randomized control trials, such as importance of infrastructure, recruiting broad population, local healthcare professionals and careful study design (33).
In conclusion, improvement was obtained after symptomatic asthma patients with insufficient control by ICS/LABA were switched to FF/VI at the equivalent corticosteroid dose. FF/VI may be a useful option for better treatment of asthma in the real-world setting because of its high clinical efficacy, long duration of activity, and delivery via a single-action device.
Acknowledgments
The authors are grateful to Prof. Yoshiki Ishii, Department of Respiratory and Clinical Immunology Dokkyo University School of Medicine, for useful discussion on study design and results, and to Reiko Komura, Department of Respiratory and Clinical Immunology Dokkyo University School of Medicine, for measuring FeNO.
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki. The Human Research Committee of Dokkyo University approved this study and it was registered as clinical trial number C-274-03. All patients gave written informed consent before enrollment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2019. Available online: www.ginasthma.org
- Matsunaga K, Hamada K, Oishi K, et al. Factors Associated with Physician-Patient Discordance in the Perception of Asthma Control. J Allergy Clin Immunol Pract 2019;7:2634-41. [Crossref] [PubMed]
- Colice GL, Ostrom NK, Geller DE, et al. The CHOICE survey: high rates of persistent and uncontrolled asthma in the United States. Ann Allergy Asthma Immunol 2012;108:157-62. [Crossref] [PubMed]
- Gold LS, Montealegre F, Allen-Ramey FC, et al. Level of asthma control and healthcare utilization in Latin America. Allergy 2013;68:1463-6. [Crossref] [PubMed]
- Demoly P, Annunziata K, Gubba E, et al. Repeated cross-sectional survey of patient-reported asthma control in Europe in the past 5 years. Eur Respir Rev 2012;21:66-74. [Crossref] [PubMed]
- Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med 2011;105:930-8. [Crossref] [PubMed]
- Boulet LP, Vervloet D, Magar Y, et al. Adherence: the goal to control asthma. Clin Chest Med 2012;33:405-17. [Crossref] [PubMed]
- Breekveldt-Postma NS, Koerselman J, Erkens JA, et al. Treatment with inhaled corticosteroids in asthma is too often discontinued. Pharmacoepidemiol Drug Saf 2008;17:411-22. [Crossref] [PubMed]
- Williams LK, Peterson EL, Wells K, et al. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. J Allergy Clin Immunol 2011;128:1185-1191.e2. [Crossref] [PubMed]
- Wells KE, Peterson EL, Ahmedani BK, et al. Real-world effects of once vs greater daily inhaled corticosteroid dosing on medication adherence. Ann Allergy Asthma Immunol 2013;111:216-20. [Crossref] [PubMed]
- Chiu KC, Chou YL, Hsu JY, et al. Comparison of the efficacy of ciclesonide with that of budesonide in mild to moderate asthma patients after step-down therapy: a randomised parallel-group study. NPJ Prim Care Respir Med 2014;24:14010. [Crossref] [PubMed]
- New JP, Bakerly ND, Leather D, et al. Obtaining real-world evidence: the Salford Lung Study. Thorax 2014;69:1152-54. [Crossref] [PubMed]
- Woodcock A, Vestbo J, Bakerly ND, et al. Effectiveness of fluticasone furoate plus vilanterol on asthma control in clinical practice: an open-label, parallel group, randomised controlled trial. Lancet 2017;390:2247-55. [Crossref] [PubMed]
- Jacques L, Bakerly ND, New JP, et al. Effectiveness of fluticasone furoate/vilanterol versus fluticasone propionate/salmeterol on asthma control in the Salford Lung Study. J Asthma 2019;56:748-57. [Crossref] [PubMed]
- Watanabe T, Chibana K, Shiobara T, et al. Expression of intelectin-1 in bronchial epithelial cells of asthma is correlated with T-helper 2 (Type-2) related parameters and its function. Allergy Asthma Clin Immunol 2017;13:35. [Crossref] [PubMed]
- Sato Y, Chibana K, Horigane Y, et al. Comparison of inducible nitric oxide synthase mRNA expression in different airway portions and association with nitric oxide parameters from patients with asthma. Clin Exp Allergy 2019;49:582-90. [Crossref] [PubMed]
- Shimizu Y, Kamiyoshihara M, Okajo J, et al. Tracheobronchial stenosis evaluated by inspiratory and expiratory three-dimensional computed tomography and impulse oscillation with three-dimensional color imaging in a patient with relapsing polychondritis. J Biol Regul Homeost Agents 2014;28:325-31. [PubMed]
- Dwan K, Milan SJ, Bax L, et al. Vilanterol and fluticasone furoate for asthma. Cochrane Database Syst Rev 2016;9:CD010758. [PubMed]
- Menzies-Gow A, Mansur AH, Brightling CE. Clinical utility of fractional exhaled nitric oxide (FeNO) in severe asthma management. Eur Respir J 2020;55:1901633. [Crossref] [PubMed]
- Ohta K, Ichinose M, Nagase H, et al. Japanese Guideline for Adult Asthma 2014. Allergol Int 2014;63:293-333. [Crossref]
- Herland K, Akselsen JP, Skjønsberg OH, et al. How representative are clinical study patients with asthma or COPD for a larger "real life" population of patients with obstructive lung disease? Respir Med 2005;99:11-9. [Crossref] [PubMed]
- Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med 2004;170:836-44. [Crossref] [PubMed]
- Adams RJ, Fuhlbrigge A, Guilbert T, et al. Inadequate use of asthma medication in the United States: results of the asthma in America national population survey. J Allergy Clin Immunol 2002;110:58-64. [Crossref] [PubMed]
- Stanford RH, Averell C, Parker ED, et al. Assessment of Adherence and Asthma Medication Ratio for a Once-Daily and Twice-Daily Inhaled Corticosteroid/Long-Acting β-Agonist for Asthma. J Allergy Clin Immunol Pract 2019;7:1488-1496.e7. [Crossref] [PubMed]
- Derendorf H, Meltzer EO. Molecular and clinical pharmacology of intranasal corticosteroids: clinical and therapeutic implications. Allergy 2008;63:1292-300. [Crossref] [PubMed]
- Slack RJ, Barrett VJ, Morrison VS, et al. In vitro pharmacological characterization of vilanterol, a novel long-acting β2-adrenoceptor agonist with 24-hour duration of action. J Pharmacol Exp Ther 2013;344:218-30. [Crossref] [PubMed]
- Woodcock A, Bleecker ER, Lötvall J, et al. Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: a randomized trial. Chest. 2013;144:1222-9. [Crossref] [PubMed]
- Bardsley G, Daley-Yates P, Baines A, et al. Anti-inflammatory duration of action of fluticasone furoate/vilanterol trifenatate in asthma: a cross-over randomised controlled trial. Respir Res 2018;19:133. [Crossref] [PubMed]
- Kharitonov SA, Donnelly LE, Montuschi P, et al. Dose-dependent onset and cessation of action of inhaled budesonide on exhaled nitric oxide and symptoms in mild asthma. Thorax 2002;57:889-96. [Crossref] [PubMed]
- Silkoff PE, McClean P, Spino M, et al. Dose-response relationship and reproducibility of the fall in exhaled nitric oxide after inhaled beclomethasone dipropionate therapy in asthma patients. Chest 2001;119:1322-8. [Crossref] [PubMed]
- van Rensen EL, Straathof KC, Veselic-Charvat MA, et al. Effect of inhaled steroids on airway hyperresponsiveness, sputum eosinophils, and exhaled nitric oxide levels in patients with asthma. Thorax 1999;54:403-8. [Crossref] [PubMed]
- Svedsater H, Jacques L, Goldfrad C, et al. Ease of use of the ELLIPTA dry powder inhaler: data from three randomised controlled trials in patients with asthma. NPJ Prim Care Respir Med 2014;24:14019. [Crossref] [PubMed]
- Leather DA, Jones R, Woodcock A, et al. Real-World Data and Randomised Controlled Trials: The Salford Lung Study. Adv Ther 2020;37:977-97. [Crossref] [PubMed]


