
Correlation between fractional exhaled nitric oxide and Asthma Control Test score and spirometry parameters in on-treatment-asthmatics in Ho Chi Minh City
Introduction
Asthma is a chronic inflammatory disease of the airway. The aim of asthma management is to control the background inflammation and allow patients to achieve and maintain asthma control (1). In asthma, the prominent inflammatory mechanism is Th2-driven inflammation (also called eosinophilic inflammation) (2-4). Among various methods used to determine and measure this type of airway inflammation, fractional exhaled nitric oxide (FeNO) is currently the simplest and most reliable tool in clinical practice (5,6). In 2011, the American Thoracic Society (ATS) published guidelines for the use of FeNO, stating that FeNO is a simple, quantitative, noninvasive and safe tool to measure airway eosinophilic inflammation in asthma (7).
Currently, clinicians always consider factors related to asthma control and asthma severity to support clinical decision-making in asthma management. For asthma control in Vietnam, besides use of the Global Initiative for Asthma (GINA) criteria, the Asthma Control Test (ACT) questionnaire is most frequently used to assess asthma control and is validated for use in Vietnam (8,9). For asthma severity, although GINA (from 2014 onwards) recommends to use their treatment steps to determine patients’ asthma severity, spirometry is still used in many countries (including Vietnam) as an important indicator for potential adverse outcomes and for information on asthma severity (10,11). To evaluate the role of FeNO in asthma management, the relationship between FeNO and asthma control and asthma severity was tested and an association was found between FeNO and the ACT score, as well between FeNO and spirometry parameters (12-19). However, no data are available to confirm whether this relationship also exists in asthmatic patients in Vietnam.
Therefore, the present study aimed to determine possible correlations between FeNO and the ACT score and between FeNO and patients’ spirometry parameters.
Methods
Study design and setting
This was a prospective cross-sectional study that involved 410 eligible participants who are ambulatory patients and recruited between March 2016 and March 2017 at the Asthma & COPD Clinic, University Medical Center, Ho Chi Minh City, Vietnam.
Ethical approval
The study protocol was approved by the Institutional Review Board of the University of Medicine & Pharmacy at Ho Chi Minh City, Vietnam. All patients and authorized representatives were given a written informed consent and those who participated or their representative in this study had to sign this consent form.
Inclusion and exclusion criteria
Patients that were eligible for inclusion in this study were (I) aged ≥12 years, (II) diagnosed at least 6 months previously with asthma according to GINA 2015 (had asthma symptoms and evidence of bronchodilator reversibility test—FEV1 change ≥12% and 200 mL after inhale 4 puffs (400 mcg salbutamol) of Ventolin®), (III) treated and followed-up by doctors at this clinic, and (IV) had sufficient command of the Vietnamese language to respond to questionnaires.
Excluded were patients meeting any of the following exclusion criteria: (I) diagnosed with allergic rhinitis and other skin atopic conditions, (II) hospitalized for asthma or had an acute upper or lower respiratory tract infection within 4 weeks prior to this study; (III) had a known respiratory disorder other than asthma and/or systemic/thoracic abnormalities that might influence normal lung function; (IV) currently smoking or had smoked >10 pack-years; (V) did not adhere to their treatment more than 2 weeks within 3 months prior to the study.
Sample size and sampling technique
Sample size required to determine whether a correlation coefficient differs from zero was calculated from this formula (20): N=[(Zα+Zβ)/C]2 +3
With α (two-tailed) =0.05 (Type I error rate), β =0.20 (Type II error rate), C =0.5*ln[(1+r)/(1−r)] and estimated correlation coefficient r=0.15 → n=347. The higher the correlation coefficient, the lower the sample size.
Data collection
- Asthma Control Test (ACT): this test comprises five questions assessing the frequency of shortness of breath, frequency of asthma nighttime symptoms, degree of functional limitation, frequency of using rescuers, and patient’s self-assessment of their level of asthma control. Each item has five response choices (each with a score ranging from 1–5). Accordingly, the level of asthma control is categorized as follows: controlled (scores 20–25), partially controlled (scores 15–19), and uncontrolled (scores <15) (8). The Vietnamese version of the ACT questionnaire has been validated (8) and was used in this study.
- FeNO measurement: FeNO was measured by a Niox Mino device (Aerocrine AB, Solna, Sweden) at flow rate of 50 mL/s for 10 seconds, according to the user’s manual (21,22). FeNO measurement was performed according to the ATS/European Respiratory Society (ERS) 2005 recommendations, Single-Breath online measurement with flow rate of 50 mL/s (23). Participants who were indicated for FeNO measurement underwent this test before performing the spirometry test to avoid distort the spirometry's results.
- Spirometry: this was conducted using a Koko spirometer (nSpire Health, USA) following the manufacturer’s instructions (24). Calibration of the device and and preparation of the patients before measurement was in accordance with the ERS/ATS 2005 recommendations (25). Participants performed spirometry after FeNO measurement. Spirometry variables comprised FVC% predicted (%FVC), FEV1% predicted (%FEV1), FEV1/FVC, PEF% predicted (%PEF), and FEF25–75% predicted (%FEF25–75).
Statistical analysis
Data were processed with Epidata software and analyzed using STATA 12.0 software. Ratio variables are presented as mean and standard deviation (SD). Student’s t-test was used to compare the means of two groups and one-way ANOVA to compare the means of multiple groups for normally distributed data. Mann-Whitney U-test and Kruskal Wallis test were used to compare the median of two groups or of more than two groups, respectively, for non-normally distributed data. Correlations between FeNO and the other outcomes were analyzed using Spearman’s correlation test. A P value ≤0.05 was considered statistically significant. In this study, the results showed that FeNO was not associated with age, gender, BMI, duration of disease, current respiratory symptoms, history of cigarette smoking, family history of allergy and trigger factors. Thus, it seems that the influence of these epidemiological features on the correlation between FeNO and ACT or FeNO and spirometry was not much then multivariate regression was not used in this analysis.
Results
Characteristics of study population
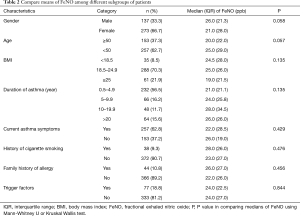
Of the 435 patients participated to the study, 25 (6%) were excluded from the analysis because of the following reasons: 8 patients failed to perform standard spirometry (did not meet ATS/ERS criterion A, B, C and D) (25-27); 9 patients failed to be measured FeNO; 2 patients did not know how to respond to ACT questionnaire and 6 patients were suspected to have asthma and COPD overlap (ACO). There were 410 participants whose data were analyzed with mean age of 42 (range, 12–76) years and 65% were female (Table 1). The mean and median ACT score were 20.5 and 21.0; mean and median FeNO were 29.5 and 24.0 parts per billion (ppb). Additional functional and biological patient characteristics are presented in Table 1. FeNO was divided into three groups according to the ATS category, i.e., mild (<25 ppb), moderate (25–50 ppb) and high (>50 ppb) (7) and the percentages of these groups were 52%, 33% and 15%, respectively. Epidemiological features of asthmatic patients (such as age, gender, BMI, duration of the disease, current respiratory symptoms, history of cigarette smoking, family history of allergy and trigger factors) may affect to FeNO results so they can influence to the correlation between FeNO and ACT or between FeNO and spirometry parameters. Therefore, a comparison of means of FeNO among subgroups of patients with different epidemiological features was presented in Table 2.

Full table
Full table
FeNO was not associated with age, gender, BMI, duration of disease, current respiratory symptoms, history of cigarette smoking, history of allergy and trigger factors (all P>0.05).
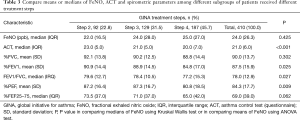
Treatment may affect all main variables such as FeNO, ACT and spirometry indexes; therefore Table 3 was created to determine this influence. FeNO is not different among three groups received step 2, 3 and 4 of GINA treatment but ACT and obstructive spirometric parameters (%FEV1, FEV1/FVC and %PEF) are significantly different among these three groups.
Full table
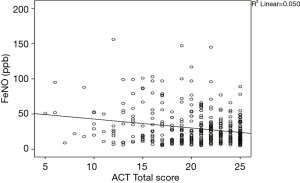
Correlations between FeNO and the ACT score are presented in Figure 1; Spearman’s correlation coefficient was r=−0.224 (P<0.001). Spearman’s correlation coefficients for FeNO and the spirometry variables were displayed in Table 4. However, bronchodilators such as LABAs may influence in airflow limitation and so distort these correlations, therefore a subgroups analysis with and without LABA component in patients’ treatment were investigated. In LABA subgroup, all obstructive spirometric parameters (%FEV1, FEV1/FVC, %PEF, %FEF25–75) but not restrictive spirometric parameter (%FVC) correlated with FeNO and these correlations are similar to correlations in the whole study population. However, the correlations in non-LABA subgroups are opposite with correlations in LABA subgroup.

Full table
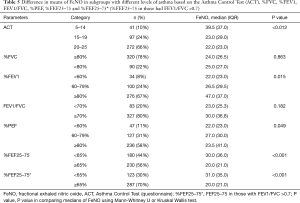
Table 5 shows differences in mean FeNO in the subgroups of patients with different levels of spirometry variables (categorized as normal vs. abnormal; or as levels of abnormality). Medians of FeNO are significantly different in subgroups of asthmatic patients having different ACT scores, %FEV1, %PEF and %FEF25–75 (%FEF25–75 in both scenarios e.g., in whole participants and in those who have FEV1/FVC >0.7). Because FEF25–75 can represent small airway airflow obstruction in an early stage before FEV1 and FEV1/FVC become abnormal, and because FeNO also measures airway inflammation in asthma associated with small airway obstruction (28), Tables 4 and 5 (in addition to data on the whole participants) also show the relationship between FeNO and %FEF25–75 as determined in patients with no obstructive syndrome (i.e., Gaensler index, FEV1/FVC >0.7).
Full table
Discussion
The results of this study showed that FeNO was not associated with many epidemiological features of asthmatic patients such as age, gender, BMI, duration of the disease, current respiratory symptoms, history of cigarette smoking and family history of allergy and having trigger factors (Table 2). This finding was supported by many previous studies although some others had opposite results. For example, one study in Vietnam found that FeNO in healthy people was not associated with epidemiological factors of the participants such as age, gender, weight, BMI except a fair correlation with height (29). Regarding the relationship between FeNO and genders, Olivieri and colleagues found that there was a difference between the sexes in which women had lower FeNO than men, and this finding was reported in studies (30-38). On the other hand, many other authors found that this relationship did not exist (29,39,40). In term of age, studies showed that there was a correlation between FeNO and age in children (18,32,41-44) but not in adults (7,30,35,36,40). Allergic symptoms were proven to increase FeNO in patients with and without asthma; however, this statement is not always true (7,45). In a study of adult patients, Kalpaklioglu found no difference in the levels of oral FeNO among patients with allergic rhinitis, with non-allergic rhinitis, or healthy persons. In addition, FeNO levels are higher in patients having non-allergic rhinitis with asthma than those having allergic rhinitis with asthma (46). With the above information, FeNO results seem not to be much interfered by many epidemiological features of asthmatic patients, thus ATS 2011 recommended using threshold of FeNO rather than using predictive values like in spirometry (7).
In the present study, most patients (66%) were categorized as having well controlled asthma based on their ACT scores (ACT 20–25) and only 10% had uncontrolled asthma (ACT 5–14) (Table 5). However, the relatively high mean FeNO (29.5 ppb) of 410 on treated patients (with GINA step 2–4) indicates that airway inflammation still occurs in asthmatic patients receiving treatment according to the GINA guidelines. This finding is consistent with earlier observations. For example, Gemicioglu et al. investigated 416 on-treatment-asthmatics and found that mean FeNO was 31.8 ppb using the same measurement device as used in the present study (Niox Mino) and with a similar ACT score (19 vs. 20.5 in the present study) (32). In addition, our FeNO data are similar to other studies worldwide investigating on-treatment-asthmatics: e.g., 31.5 ppb in the USA (47), 31 ppb in Nepal (48) and 38.4 ppb recently reported in India (16).
The mean FeNO in well-controlled asthmatic patients in the present study (26.9 ppb, not presented in result part) was higher than the lower threshold of the ATS categorization (<25 ppb) (7), this result and other reports indicated that it is difficult to completely control airway inflammation by optimal intervention and the airway inflammation even still exist in ex-asthma patients (49,50). Because there is no comparable study on FeNO in Vietnam, no direct comparison can be made with an asthmatic Vietnamese population. However, previous studies on FeNO in Vietnam are mentioned here to provide a brief overview of FeNO levels in this land. For example, based on two small studies (conducted in the middle and the south of Vietnam), mean FeNO ranged from 10.4 to 15.7 ppb in healthy persons (29,51) compared to 31.1 ppb (51) and 18.8 ppb (51) in ACO and COPD patients, respectively. These comparisons imply that FeNO in treated asthmatics is much higher than that of COPD patients or of healthy persons. These findings confirm previous reports showing that airway inflammation cannot be completely suppressed by optimal treatment, or that inflammation persists even in ex-asthmatics (asthmatics had no abnormal symptoms and signs and had no treatment) (49,50).
A negative and weak correlation was found between FeNO and the ACT score (Spearman's r =−0.224, P<0.001); in addition, mean FeNO level increases when ACT-based asthma control levels deteriorate (Table 5). This means that FeNO can reflect asthma control (as also reported in many studies). The negative and weak correlation between FeNO and ACT score exists both in steroid naive asthmatics and in asthmatics-on-treatment. In steroid naive patients, some correlations reported by others researchers are stronger than ours, e.g., Senna et al. (12) (n=27, r =−0.69, P=0.001), Bernstein et al. (14) (n=55, r =−0.48, P<0.001), Mohan et al. (52) (n=96, r =−0.75, P<0.001) and Kavitha et al. (16) (n=151, r =−0.76, P<0.001). In treated patients, similar to the present population, the correlations are much weaker than in the naive group, e.g., Shirai et al. (15) (n=105, r =−0.31; P=0.003), Gutierrez et al. (53) (n=441, r =−0.16; P<0.01), Habib et al. (54) (n=53, r =−0.581; P<0.0001), Gemicioglu et al. (32) (n=416, r =−0.31; P=0.002), Mohan et al. (52) (n=96, r =−0.65; P<0.001) and Kavitha et al. (16) (n=151, r =−0.68; P<0.001). However, other groups found no correlation between these two variables. For example, Han et al. (55) and Yangui et al. (56) found no correlation whereas Bernstein et al. (14) found this association in their untreated group but not in their treated group. In Vietnam, a unique study including 42 children found no correlation between FeNO and the other variables investigated (57). The present study found that FeNO value was higher in the groups with lower levels of asthma control. Similarly, Papakosta et al., Shirai et al. and Habib et al. also reported that the means/medians of FeNO showed a significant difference between the two/three groups with different asthma control levels, with higher FeNO in the worse asthma control group (13,15,54).
In asthma, chronic airway inflammation can result in chronic airflow limitation. Whereas airway inflammation plays a key role in the pathogenesis of asthma, it is less clear whether poor lung function is associated with severe inflammation. Although some studies found an association between higher levels of inflammatory markers and more severe airflow limitation, there is no consensus (11,12,58-60). The spirometry parameters %FEV1 and %FEF25–75 have received increasing attention regarding this association (12,59). The present study found that almost all variables related to airway obstruction, e.g., %FEV1, %PEF, %FEF25–75 and the Gaensler ratio (FEV1/FVC), had a significant correlation with FeNO. However, the parameter related to airway restriction (%FVC) was not correlated with FeNO (Table 4). This finding is similar to result in another Vietnamese study in which FeNO have a significant association with FEV1, FEV1/FVC and PEF (61). Contrary to the present study’s results, Gemicioglu et al. found no association between FeNO with any spirometry parameters in 2 recent studies (32,44). However, in the present study, when subgroups of patients with and without LABA component in their treatment was analyzed, the correlations between FeNO and obstructive spirometric indexes (%FEV1, Gaensler ratio, %PEF, %FEF25–75) only happen in LABA treatment group but not in non-LABA treatment group and vice versa with restrictive index (%FVC).
Regarding FeNO and %FEV1, Kavitha et al. reported that FeNO had a strong correlation with FEV1 (n=151, r =−0.78, P<0.001) (16) and Torre et al. (17), Leung et al. (18) and Surja et al. (62) found that FeNO correlated with FEV1 with coefficients of r =−0.2 (n=96, P=0.03), r =−0.221 (n=92, P=0.014) and r2=0.403 (n=56, P=0.001), respectively. In pregnant women, Nittner-Marszalska et al. also found a correlation between FeNO and FEV1 (n=72, r =−0.21; P=0.0014) (19). Nevertheless, many researchers reported that no such association was found such as Senna (n=27, r =−0.24, P=0.23) (12), Yangui (n=37, r =−0.02, P>0.05) (56), Silkoff (63), Xia (n=57, r =−0.186, P>0.05) (64), Zietkowski (n=101, r =0.02, P=0.87) (59), Dal Negro (n=20, r =−0.38, P>0.05) (65) and Stirling (n=52, P=0.73) (66).
It is well established that inflammation in asthma involves the large airways; however, small airways are now widely accepted as a major site of inflammation (67). The mid-flow rates measured during spirometry testing (FEF25–75) are believed to represent small airway airflow (68,69). Since FEF25–75 is generally considered to be an approximate measure of distal airways caliber, reduced FEF25–75 is considered to represent small airways obstruction caused by asthma inflammation (70,71). FeNO is a biomarker of airway inflammation in asthma which is associated with small airway obstruction (28). Several studies found that %FEF25–75 is significantly related with FeNO (72-77). Tosca et al. reported that FeNO correlated with %FEF25–75 (n=56, r =−0.33; P=0.01) (78) and del Giudice et al. found a correlation between FeNO and %FEF25–75 (n=37, P<0.0098; r =0.439) (79). In asthmatic children, Lim et al. divided %FEF25–75 into two groups (group 1 with normal %FEF25–75, i.e., ≥65%, and group 2 with abnormal %FEF25–75 i.e., <65%) and found a correlation with FeNO in group 2 (n=28, r =−0.493, P=0.038) but not in group 1 (n=90, r =−0.037; P=0.749) (80). In adults, Malerba et al. found that FeNO was correlated with %FEF25–75 (81). However, other studies in adults, e.g., Nishimoto et al. (44) and Silkoff et al. (63), found no such correlation. The present study shows a weak correlation between FeNO and %FEF25–75; however, FeNO in the abnormal %FEF25–75 group (%FEF25–75 <65%) was significantly higher than that of the normal %FEF25–75 group (%FEF25–75≥65%) (30.0 vs. 20 ppb; P<0.001, compare FEF25–75 in Table 5).
Current guidelines recommended using FEV1 to evaluate limitation of airway in asthmatic patients (1). However, air trapping can happen in asthma patients with normal FEV1 (82) and this trapping was proved to have good correlation with FEF25–75 rather than with FEV1 (83). Therefore, correlation between FeNO and FEF25–75 was recently studied in subjects without obstruction determined by FEV1/FVC (74). In our subgroup of patients with no airway obstruction (FEV1/FVC >0.7), FeNO still showed a correlation with %FEF25–75 (r =−0.22, P<0.001); also, a significant difference was found between the two groups of normal and abnormal %FEF25–75 (31.0 vs. 20.0 ppb; P<0.001, compare FEF25–75* in Table 5). This finding is consistent with data from Malerba et al. who found that FeNO correlated with %FEF25–75 under the condition that FVC, FEV1 and FEV1/FC were all in the normal range (81). These latter authors suggested that abnormal %FEF25–75 might be considered an early marker of airflow limitation associated with eosinophilic inflammation (81).
In general, there are some conflicting evidences about the correlation between FeNO and asthma control indicators (such as ACT) or between FeNO and asthma severity indicators (such as spirometry parameters). There is not comparable study in Vietnam to compare, however, with the results in the present study, it can be concluded that these relationships exist in the Vietnamese population and this may provide some information for using FeNO in asthma management in this population.
The present study has some limitations. First, because this study was conducted in one hospital only, the results are not generalizable to other locations and/or other countries. Second, other factors that could influence FeNO levels, e.g., traffic-related pollution exposure and second-hand tobacco smoke, were not evaluated (84).
Conclusions
This study shows that FeNO is correlated with the ACT score, %FEV1 and %FEF25–75 and that there is a significant difference between subgroups categorized according to different levels of these variables. The higher the level of ACT-based asthma control, the lower the FeNO (and vice versa); also, FeNO stability increases when %FEV1, %FEF25–75 worsens.
In view of these findings, together with the fact that FeNO measurement is simple, noninvasive and easy to interpret, this parameter may well be a useful tool for asthma management in clinical practice.
Acknowledgments
The authors acknowledge Staff in Department of Respiratory Functional Exploration, University Medical Center, Ho Chi Minh City, Vietnam.
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.04.01). VNN reports grants from University of Medicine and Pharmacy at Ho Chi Minh City, Viet Nam, other from Aerocrine Company, during the conduct of the study. NHC has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Institutional Review Board of the University of Medicine & Pharmacy at Ho Chi Minh City, Vietnam. All patients and authorized representatives were given a written informed consent and those who participated or their representative in this study had to sign this consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Initiative for Asthma. Global Strategy For Asthma Management And Prevention (GINA), updated 2017. 2017. Available online: www.ginasthma.org. Accessed September 5, 2019.
- Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009;180:388-95. [Crossref] [PubMed]
- Fahy JV. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol 2015;15:57-65. [Crossref] [PubMed]
- Murdoch JR, Lloyd CM. Chronic inflammation and asthma. Mutat Res 2010;690:24-39. [Crossref] [PubMed]
- Kim MA, Shin YS. Adult asthma biomarkers. Curr Opin Allergy Clin Immunol 2014;14:49-54. [Crossref] [PubMed]
- Warke TJ, Fitch PS, Brown V, et al. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax 2002;57:383-7. [Crossref] [PubMed]
- Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602-15. [Crossref] [PubMed]
- Nguyen VN, Chavannes N, Le LT, et al. The Asthma Control Test (ACT) as an alternative tool to Global Initiative for Asthma (GINA) guideline criteria for assessing asthma control in Vietnamese outpatients. Prim Care Respir J 2012;21:85-9. [Crossref] [PubMed]
- Nguyen VN, Nguyen QN, Le An P, et al. Implementation of GINA guidelines in asthma management by primary care physicians in Vietnam. Int J Gen Med 2017;10:347-55. [Crossref] [PubMed]
- Chhabra SK. Clinical application of spirometry in asthma: Why, when and how often? Lung India 2015;32:635-7. [Crossref] [PubMed]
- Schifano ED, Hollenbach JP, Cloutier MM. Mismatch between Asthma Symptoms and Spirometry: Implications for Managing Asthma in Children. J Pediatr 2014;165:997-1002. [Crossref] [PubMed]
- Senna G, Passalacqua G, Schiappoli M, et al. Correlation among FEV, nitric oxide and asthma control test in newly diagnosed asthma. Allergy 2007;62:207-8. [Crossref] [PubMed]
- Papakosta D, Latsios D, Manika K, et al. Asthma control test is correlated to FEV1 and nitric oxide in Greek asthmatic patients: influence of treatment. J Asthma 2011;48:901-6. [Crossref] [PubMed]
- Bernstein JA, Davis B, Alvarez-Puebla MJ, et al. Is exhaled nitric oxide a useful adjunctive test for assessing asthma? J Asthma 2009;46:955-60. [Crossref] [PubMed]
- Shirai T, Furuhashi K, Suda T, et al. Relationship of the asthma control test with pulmonary function and exhaled nitric oxide. Ann Allergy Asthma Immunol 2008;101:608-13. [Crossref] [PubMed]
- Kavitha V, Mohan A, Madan K, et al. Fractional exhaled nitric oxide is a useful adjunctive modality for monitoring bronchial asthma. Lung India 2017;34:132-7. [Crossref] [PubMed]
- Torre O, Olivieri D, Barnes PJ, et al. Feasibility and interpretation of FE(NO) measurements in asthma patients in general practice. Respir Med 2008;102:1417-24. [Crossref] [PubMed]
- Leung TF, Li CY, Lam CW, et al. The relation between obesity and asthmatic airway inflammation. Pediatr Allergy Immunol 2004;15:344-50. [Crossref] [PubMed]
- Nittner-Marszalska M, Liebhart J, Pawlowicz R, et al. Fractioned exhaled nitric oxide (FE(NO)) is not a sufficiently reliable test for monitoring asthma in pregnancy. Nitric Oxide 2013;33:56-63. [Crossref] [PubMed]
- Explore the Training in Clinical Research Program at UCSF. Sample size calculators for designing clinical research. Available online: https://www.sample-size.net/correlation-sample-size/. Accessed on January 20 2020.
- Aerocrine. User manual 000164 (EPM-000109), version 9. 2014. Available online: http://www.niox.com/Documents/000164-09%20NIOX%20MINO%20User%20Manual%20(US).pdf. Accessed on 1 October 2018.
- Aerocrine. NIOX MINO External Quality Control User Manual. 2016. Available online: http://www.niox.com/Documents/000164-09%20NIOX%20MINO%20User%20Manual%20(US).pdf. Accessed 01 October 2018.
- American Thoracic Society. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171:912-30. [Crossref] [PubMed]
- nSpire Health. KoKo® Spirometer. KoKo DigiDoserTM. Operations Guide. 2007. Available online: http://respitechservice.com/Files-hold/KoKo%20User%20Manual.pdf. Accessed 01 October 2018.
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Health n. KoKo® Spirometer. KoKo DigiDoserTM. Operations Guide. Available online: http://respitechservicecom/Files-hold/KoKo%20User%20Manualpdf Accessed on 24 October 2017 2007.
- Zuo WL, Shenoy SA, Li S, et al. Ontogeny and Biology of Human Small Airway Epithelial Club Cells. Am J Respir Crit Care Med 2018;198:1375-88. [Crossref] [PubMed]
- Battaglia S, den Hertog H, Timmers MC, et al. Small airways function and molecular markers in exhaled air in mild asthma. Thorax 2005;60:639-44. [Crossref] [PubMed]
- Duong QS, Nguyen HTP, Nguyen NV, et al. Study of exhaled nitric oxide concentration in healthy Vietnamese. Ho Chi Minh City Medical Journal 2012;16:22-9. Available online: http://yds.edu.vn/tcyh/?Content=ChiTietBai&idBai=9972. Accessed on 01 December 2019.
- Olivieri M, Talamini G, Corradi M, et al. Reference values for exhaled nitric oxide (reveno) study. Respir Res 2006;7:94. [Crossref] [PubMed]
- Taylor DR, Mandhane P, Greene JM, et al. Factors affecting exhaled nitric oxide measurements: the effect of sex. Respir Res 2007;8:82. [Crossref] [PubMed]
- Gemicioglu B, Musellim B, Dogan I, et al. Fractional exhaled nitric oxide (FeNo) in different asthma phenotypes. Allergy Rhinol (Providence) 2014;5:157-61. [Crossref] [PubMed]
- Grasemann H. Effects of sex and of gene variants in constitutive nitric oxide synthases on exhaled nitric oxide. Am J Respir Crit Care Med 2003;167:1113-6. [Crossref] [PubMed]
- Jilma B, Kastner J, Mensik C, et al. Sex differences in concentrations of exhaled nitric oxide and plasma nitrate. Life Sci 1996;58:469-76. [Crossref] [PubMed]
- Travers J, Marsh S, Aldington S, et al. Reference ranges for exhaled nitric oxide derived from a random community survey of adults. Am J Respir Crit Care Med 2007;176:238-42. [Crossref] [PubMed]
- Tsang KW, Ip SK, Leung R, et al. Exhaled nitric oxide: the effects of age, gender and body size. Lung 2001;179:83-91. [Crossref] [PubMed]
- van der Lee I, van den Bosch JM, Zanen P. Reduction of variability of exhaled nitric oxide in healthy volunteers. Respir Med 2002;96:1014-20. [Crossref] [PubMed]
- Nguyen DT, Kit BK, Brody D, et al. Prevalence of high fractional exhaled nitric oxide among US youth with asthma. Pediatr Pulmonol 2017;52:737-45. [Crossref] [PubMed]
- Olin AC, Bake B, Toren K. Fraction of exhaled nitric oxide at 50 mL/s: reference values for adult lifelong never-smokers. Chest 2007;131:1852-6. [Crossref] [PubMed]
- Olin AC, Rosengren A, Thelle DS, et al. Height, age, and atopy are associated with fraction of exhaled nitric oxide in a large adult general population sample. Chest 2006;130:1319-25. [Crossref] [PubMed]
- Buchvald F, Baraldi E, Carraro S, et al. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol 2005;115:1130-6. [Crossref] [PubMed]
- Franklin PJ, Taplin R, Stick SM. A community study of exhaled nitric oxide in healthy children. Am J Respir Crit Care Med 1999;159:69-73. [Crossref] [PubMed]
- Barros R, Moreira A, Fonseca J, et al. Obesity and airway inflammation in asthma. J Allergy Clin Immunol 2006;117:1501-2. [Crossref] [PubMed]
- Nishimoto K, Karayama M, Inui N, et al. Relationship between fraction of exhaled nitric oxide and airway morphology assessed by three-dimensional CT analysis in asthma. Sci Rep 2017;7:10187. [Crossref] [PubMed]
- Grob NM, Dweik RA. Exhaled nitric oxide in asthma. From diagnosis, to monitoring, to screening: are we there yet? Chest 2008;133:837-9. [Crossref] [PubMed]
- Kalpaklioglu AF, Kalkan IK. Comparison of orally exhaled nitric oxide in allergic versus nonallergic rhinitis. Am J Rhinol Allergy 2012;26:e50-4. [Crossref] [PubMed]
- Ritz T, Kullowatz A, Bill MN, et al. Daily life negative mood and exhaled nitric oxide in asthma. Biol Psychol 2016;118:176-83. [Crossref] [PubMed]
- Shrestha SK, Shrestha S, Sharma L, et al. Comparison of fractional exhaled nitric oxide levels in chronic obstructive pulmonary disease, bronchial asthma and healthy subjects of Nepal. J Breath Res 2017;11:047101. [Crossref] [PubMed]
- Hanxiang N, Jiong Y, Yanwei C, et al. Persistent airway inflammation and bronchial hyperresponsiveness in patients with totally controlled asthma. Int J Clin Pract 2008;62:599-605. [Crossref] [PubMed]
- Ward C, Pais M, Bish R, et al. Airway inflammation, basement membrane thickening and bronchial hyperresponsiveness in asthma. Thorax 2002;57:309-16. [Crossref] [PubMed]
- Vinh NN, Ngoc TV, Ba NTT. Levels of fractional exhaled nitric oxide (FeNO) in asthma-copd overlap (ACO) patients compared with those in COPD patients and in healthy persons in Viet Nam. Respirology 2017;22:245. [Crossref]
- Mohan A, Kavitha V, Madan K, et al. Utility of fractional exhaled nitric oxide (FeNO) in assessing asthma control following inhaled corticosteroid treatment. Eur Respir J 2016;48. [Crossref]
- Alvarez-Gutierrez FJ, Medina-Gallardo JF, Perez-Navarro P, et al. Comparison of the Asthma Control Test (ACT) with lung function, levels of exhaled nitric oxide and control according to the Global Initiative for Asthma (GINA). Arch Bronconeumol 2010;46:370-7. [Crossref] [PubMed]
- Habib SS, Alzoghaibi MA, Abba AA, et al. Relationship of the Arabic version of the asthma control test with ventilatory function tests and levels of exhaled nitric oxide in adult asthmatics. Saudi Med J 2014;35:397-402. [PubMed]
- Han CH, Park Y-I, Kwak HJ, et al. Relationship between Exhaled Nitric Oxide and Levels of Asthma Control in Asthma Patients Treated with Inhaled Corticosteroid. Tuberc Respir Dis 2011;71:106-13. [Crossref]
- Yangui F, Abouda M, Triki M, et al. Asthma control test (ACT), fractionated exhaled nitric oxide (FeNO) and forced expiratory volume in 1 second (FEV1) correlation in asthma control. Eur Respir J 2012;40:2216.
- Nguyen-Thi-Bich H, Duong-Thi-Ly H, Thom VT, et al. Study of the correlations between fractional exhaled nitric oxide in exhaled breath and atopic status, blood eosinophils, FCER2 mutation, and asthma control in Vietnamese children. J Asthma Allergy 2016;9:163-70. [Crossref] [PubMed]
- Li AM, Lex C, Zacharasiewicz A, et al. Cough frequency in children with stable asthma: correlation with lung function, exhaled nitric oxide, and sputum eosinophil count. Thorax 2003;58:974-8. [Crossref] [PubMed]
- Zietkowski Z, Bodzenta-Lukaszyk A, Tomasiak MM, et al. Comparison of exhaled nitric oxide measurement with conventional tests in steroid-naive asthma patients. J Investig Allergol Clin Immunol 2006;16:239-46. [PubMed]
- Steerenberg PA, Janssen NA, de Meer G, et al. Relationship between exhaled NO, respiratory symptoms, lung function, bronchial hyperresponsiveness, and blood eosinophilia in school children. Thorax 2003;58:242-5. [Crossref] [PubMed]
- Duong Quy S. Study of exhaled nitric oxide concentration in Vietnamese asthmatic patients. Ho Chi Minh 2012;16:135-40.
- Surja S, Yu B, Codispoti CD, et al. Fractional Exhaled Nitric Oxide (FENO) Is Negatively Associated with Percent-Predicted FEV1 in Inner-City Minority Asthma Patients. J Allergy Clin Immunol 2016;137:AB107. [Crossref]
- Silkoff PE, Strambu I, Laviolette M, et al. Asthma characteristics and biomarkers from the Airways Disease Endotyping for Personalized Therapeutics (ADEPT) longitudinal profiling study. Respir Res 2015;16:142. [Crossref] [PubMed]
- Xia Q, Pan P, Wang Z, et al. Fractional exhaled nitric oxide in bronchial inflammatory lung diseases. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014;39:365-70. [PubMed]
- Dal Negro R, Micheletto C, Tognella S, et al. Assessment of inhaled BDP-dose dependency of exhaled nitric oxide and local and serum eosinophilic markers in steroids-naive nonatopic asthmatics. Allergy 2003;58:1018-22. [Crossref] [PubMed]
- Stirling R, Kharitonov S, Campbell D, et al. Increase in exhaled nitric oxide levels in patients with difficult asthma and correlation with symptoms and disease severity despite treatment with oral and inhaled corticosteroids. Thorax 1998;53:1030-4. [Crossref] [PubMed]
- Hamid Q, Song Y, Kotsimbos TC, et al. Inflammation of small airways in asthma. J Allergy Clin Immunol 1997;100:44-51. [Crossref] [PubMed]
- McFadden ER Jr, Linden DA. A reduction in maximum mid-expiratory flow rate. A spirographic manifestation of small airway disease. Am J Med 1972;52:725-37. [Crossref] [PubMed]
- Wagner EM, Liu MC, Weinmann GG, et al. Peripheral lung resistance in normal and asthmatic subjects. Am Rev Respir Dis 1990;141:584-8. [Crossref] [PubMed]
- van den Berge M, Ten Hacken NHT, Cohen J, et al. Small airway disease in asthma and COPD: clinical implications. Chest 2011;139:412-23. [Crossref] [PubMed]
- Burgel PR. The role of small airways in obstructive airway diseases. Eur Respir Rev 2011;20:23-33. [Crossref] [PubMed]
- Ciprandi G, Capasso M, Leonardi S, et al. Impaired FEF25-75 values may predict bronchial reversibility in allergic children with rhinitis or asthma. J Biol Regul Homeost Agents 2012;26:S19-25. [PubMed]
- Ciprandi G, Tosca MA, Castellazzi AM, et al. FEF(25-75) might be a predictive factor for bronchial inflammation and bronchial hyperreactivity in adolescents with allergic rhinitis. Int J Immunopathol Pharmacol 2011;24:17-20. [Crossref] [PubMed]
- Yoon J-Y, Woo S-I, Kim H, et al. Fractional exhaled nitric oxide and forced expiratory flow between 25% and 75% of vital capacity in children with controlled asthma. Korean J Pediatr 2012;55:330-6. [Crossref] [PubMed]
- Spallarossa D, Battistini E, Silvestri M, et al. Steroid-naive adolescents with mild intermittent allergic asthma have airway hyperresponsiveness and elevated exhaled nitric oxide levels. J Asthma 2003;40:301-10. [Crossref] [PubMed]
- Nishio K, Odajima H, Motomura C, et al. Exhaled nitric oxide and exercise-induced bronchospasm assessed by FEV1, FEF25-75% in childhood asthma. J Asthma 2007;44:475-8. [Crossref] [PubMed]
- Simon MR, Chinchilli VM, Phillips BR, et al. FEF(25-75) and FEV(1)/FVC in Relation to Clinical and Physiologic Parameters in Asthmatic Children with Normal FEV(1) Values. The Journal of allergy and clinical immunology 2010;126:527-34. [Crossref] [PubMed]
- Tosca MA, Silvestri M, Solari N, et al. Inflammation Markers and FEF(25-75): A Relevant Link in Children With Asthma. Allergy Asthma Immunol Res 2016;8:84-5. [Crossref] [PubMed]
- del Giudice MM, Brunese FP, Piacentini GL, et al. Fractional exhaled nitric oxide (FENO), lung function and airway hyperresponsiveness in naive atopic asthmatic children. J Asthma 2004;41:759-65. [Crossref] [PubMed]
- Lim H, Kim E, Lim CH, et al. Relationships between fractional exhaled nitric oxide levels and FEF25%–75% in children with asthma. Allergy Asthma Respir Dis 2016;4:14. [Crossref]
- Malerba M, Radaeli A, Olivini A, et al. Association of FEF25-75% Impairment with Bronchial Hyperresponsiveness and Airway Inflammation in Subjects with Asthma-Like Symptoms. Respiration 2016;91:206-14. [Crossref] [PubMed]
- Samee S, Altes T, Powers P, et al. Imaging the lungs in asthmatic patients by using hyperpolarized helium-3 magnetic resonance: assessment of response to methacholine and exercise challenge. J Allergy Clin Immunol 2003;111:1205-11. [Crossref] [PubMed]
- de Lange EE, Altes TA, Patrie JT, et al. Evaluation of asthma with hyperpolarized helium-3 MRI: correlation with clinical severity and spirometry. Chest 2006;130:1055-62. [Crossref] [PubMed]
- Eckel SP, Berhane K, Salam MT, et al. Residential traffic-related pollution exposures and exhaled nitric oxide in the children's health study. Environ Health Perspect 2011;119:1472-7. [Crossref] [PubMed]


