Pre-emptive pain management program is associated with reduction of opioid prescription after minimally invasive pulmonary resection
Introduction
The availability of prescription opioids in the community has contributed to the opioid crisis (1). Opioids in the community commonly come from prescriptions to manage post-operative pain. Patients or patients’ families may misuse the excess opioid medications after surgery, using them for purposes other than the management of post-operative pain. Moreover, studies have shown that 3–10% of patients who are opioid-naïve prior to surgery are still taking opioids 1 year after surgery (2-4). Thus, a strategy to reduce the number of opioid prescriptions after surgery may benefit individual patients as well as the community: the strategy will decrease not only the chance of individuals becoming addicted to opioids but also the overall supply of opioids.
In the past, there were very limited options for patients to manage post-operative pain without opioids, especially with the management of pain in pulmonary resection surgery. In order to perform a pulmonary resection, a surgeon has to gain access between the ribs where there is an intercostal nerve, which can cause significant pain. There has been an evolution of both surgical techniques and medications to manage post-operative pain over the years, but opioids have been the standard discharge pain medication after pulmonary resection. Good pain management is a key aspect of pulmonary resection surgery; if patients have uncontrolled pain, then they are more likely to have pulmonary complications, such as pneumonia. The development of minimally invasive techniques for pulmonary resection, as well as medication strategies, such as the use of epidurals, have significantly helped in improving pain. At discharge, however, the usual pain management medication(s) have included an opioid, and typically a highly dependent schedule II opioid such as hydrocodone/acetaminophen.
The recent availability of long-acting local anesthetics and non-opioid IV medications, along with the adoption of enhanced recovery after surgery (ERAS) program, have made an impact in the management of post-operative pain from pulmonary resection. ERAS encompassed the development of peri-operative care to improve recovery. When we implemented the ERAS protocol in our division, we were able to discharge patients home with different narcotics that are less likely to cause dependency (5). However, as the ERAS protocol matured—especially in regard to pain management—and as we adopted robot-assisted surgery in pulmonary resections, we saw a significant improvement in patients’ pain control after pulmonary resections. At times patients would state that they did not take the opioid prescribed after surgery because it was not necessary. This feedback led us to alter our default home pain medication from opioid to non-opioid around-the-clock pain medications; we also counseled the patients about taking pain medications around the clock at home to keep pain at bay. We called this program the pre-emptive pain management program. In this study, we wanted to determine the impact of the pre-emptive pain management program in decreasing not only opioid prescriptions after surgery but also the pain level of these patients at their post-operative visits. We present the following article/case in accordance with STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-431).
Methods
The study was approved by Institutional Review Board at Houston Methodist Hospital (Pro00013680 and Pro00013298). We performed retrospective case-controlled study using prospectively collected society of thoracic surgeons (STS) data on all patients who underwent elective minimally invasive pulmonary resection from 2012 to 2018; all procedures were performed by surgeons from Houston Methodist Hospital’s Division of Thoracic Surgery. Robot assisted pulmonary resection used a port based approach with the standard “five on a dice” lung resection method described previously (6). We included all patients who had documented discharge pain medications, but we excluded diagnostic wedge procedures, emergent or urgent pulmonary resections, and pulmonary resections as part of the “two-step” procedure for cardiac sarcoma with pulmonary resection.
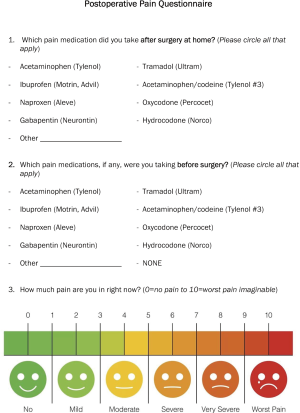
During the 6-year time period, we gradually implemented the ERAS program. In 2016, we were able to fully implement all components of the ERAS program, and in 2017 we implemented the pre-emptive pain management program. We have covered the ERAS program in detail in our previous study (5). All of the patients were managed in a similar fashion at each time period. In brief, we emphasized a total of 12 aspects in the program, which itself was divided into three phases of care: pre-operative, operative and post-operative. The pre-operative stage was developed to get the patient ready for surgery, with an emphasis on quitting smoking, learning to use the incentive spirometer, and walking 1 mile per day. The operative stage included the pre-emptive pain management program and total intravenous anesthesia, minimizing fluids and minimally invasive surgery. In addition, all patients received a direct injection of undiluted liposomal bupivacaine block of the 2nd to 10th intercostal nerves from inside of the chest cavity. The post-operative stage included an around-the-clock scheduled analgesic and emphasis on early mobility, including an incentive spirometer. We also transitioned to prescribing tramadol as a default discharge pain medication for all patients at discharge from hydrocodone/acetaminophen.
After the implementation of the ERAS program, we made two observations. First, a large number of patients had minimal pain after the removal of the chest tube prior to their discharge home. Second, some patients reported that they did not take the prescribed tramadol after surgery. These two observations made us evaluate our discharge pain medication strategy. Instead of giving patients the default opioid medication, we changed the default to an around-the-clock non-opioid medication and discussed the pain management plan prior to surgery. We called this program the pre-emptive pain management program. We describe the standard pain regimen that was used during pre-emptive pain management program with enhanced recovery after surgery (Pre-emptive), enhanced recovery after surgery program alone (ERAS) and standard care group (control) in Table 1.
Full table
In this study, we compared the outcomes of patients who were in the pre-emptive pain management program to those who were in the ERAS only group and control group. We obtained data regarding demographics, co-morbidity, procedure type, pathology, morbidity, mortality, and length of patient stay during this time period from the prospectively collected STS database and electronic health record. We also obtained information about the pain medications that patients were given at the time of discharge. We categorized these medications as opioid or non-opioid medications. We collected the number of patients in the pre-emptive pain management group who went home without opioid prescriptions, but who later called due to inadequate pain control, ultimately requiring an opioid prescription. Finally, we collected the pain level for patients at follow up visits, about 4 weeks after surgery. We ensured that we had complete data on all patients.
Demographic and clinical data were reported as frequencies and proportions for categorical variables and as median and interquartile range (IQR) for continuous variables. Differences between groups were compared using the Chi-square or Fisher’s exact tests for categorical variables and Wilcoxon rank-sum test for continuous variables as appropriate. Univariate exact logistic regression analysis and multiple logistic regression modeling were performed to determine the characteristics associated with the outcomes. Variables for the initial multiple logistic regression models were selected using the Bayesian model averaging (BMA) method and also based on the clinical importance (7,8). The likelihood test was used to reduce the model subsets. The best model was selected based on the smallest Bayesian information criterion (BIC). Model discrimination was determined by the area under the area under the receiver operating characteristic (ROC) curve (AUC). Model calibration was assessed using the Hosmer-Lemeshow goodness-of-fit test with a non-significant P value indicating good calibration. All the analyses were performed on Stata version 16.1 (StataCorp LLC, College Station, TX, USA). A P value of <0.05 was considered statistically significant.
Results
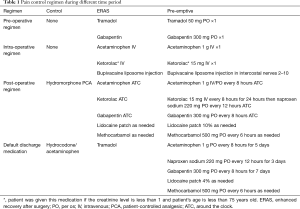
There were 443 patients who underwent elective minimally invasive pulmonary resections between 2012 and 2018 and who met the inclusion and exclusion criteria. There were 132 patients that received the pre-emptive pain management with enhanced recovery after surgery (Pre-emptive), 90 patients that received enhanced recovery after surgery alone (ERAS) and 221 patients who received standard of care (control). The median age of the groups was 66 years old, and they were predominantly female (56.4%) and white (81.7%; Table 2). The common co-morbidities were hypertension (60.7%), coronary artery disease (20.3%), diabetes (17.4), and cerebrovascular accident or TIA (10.4%). There was no significant difference in age, gender, and race or body mass index. There was a higher number of patients with diabetes in the control group than in the ERAS and pre-emptive pain control group (21.7% vs. 17.8% vs. 9.8%, P=0.02). Most patients (65.9%) underwent surgery for lung cancer. The two most common procedures were lobectomy (53%) and wedge resection (39.7%). Robot-assisted minimally invasive lung resection was performed in 50.3%. There were a significantly higher number of patients in the pre-emptive pain management group and ERAS only group compared to control who underwent robot assisted surgery (89.4% vs. 75.6% vs. 16.7%, P<0.001).
Full table
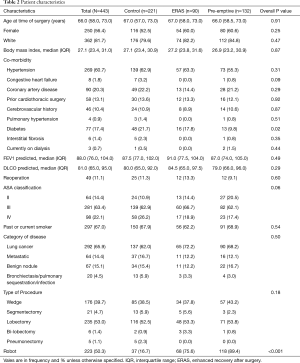
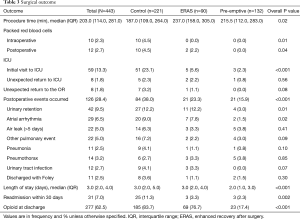
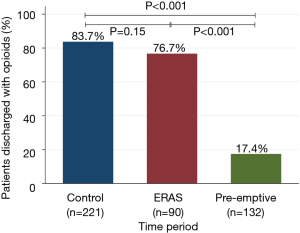
There were significantly fewer overall complications in the pre-emptive pain management group compared to ERAS group and the control group (15.9% vs. 23.3% vs. 38%, P<0.001, Table 3), with a significant decrease in urinary retention (3% vs. 12.2% vs. 12.2%, P=0.01), and atrial arrhythmia (1.5% vs. 7.8 vs. 9%, P=0.02). Overall there was less transfusions in the pre-emptive pain management group compared to ERAS group and the control group: intraoperative transfusions (0% vs. 0% vs. 4.5%, P=0.01), and postoperative transfusions (0% vs. 2.2% vs. 4.5%, P=0.04). Moreover, there was a significantly shorter median hospital length of stay (2 vs. 3 vs. 3 days, P<0.001) and 30-day readmission rate (2.3% vs. 3.3% vs. 11.3%, P=0.002) as well as fewer opioid prescriptions at discharge (17.4% vs. 76.7% vs. 83.7%, P<0.001, Figure 1) in the pre-emptive pain management group compared to the ERAS group and the control group (Table 3 and Table S1).
Full table
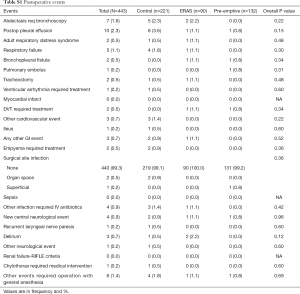
Full table
We performed univariate analysis of the factors that contribute to opioid prescriptions at discharge. We identified the pre-emptive pain control program [odds ratio (OR) 0.05; 95% confidence interval (CI), 0.03, 0.08; P<0.001], and the use of robotic technology (OR 0.17; 95% CI, 0.11, 0.26; P<0.001) as significant factors that reduced opioid prescription at discharge. We also found that non-white patients were associated with a higher rate of opioid prescriptions at discharge after a pulmonary resection (OR 1.77; 95% CI, 1.04, 3.02; P=0.04; Table 4). Using multiple logistic regression, we found that pre-emptive pain management (OR 0.06; 95% CI, 0.03, 0.11; P<0.001) and robot assisted surgery (OR 0.52; 95% CI, 0.3, 0.88; P=0.02) were the only significant factors associated with the reduction of opioid prescriptions at discharge (Table 5).

Full table
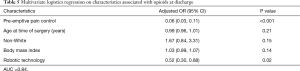
Full table
Of the 109 patients who were discharged to home without opioid prescriptions in the pre-emptive pain management group, 7% (8 patients) called with questions about pain management, but none of them required an opioid prescription. Most patients had minimal pain at 4 weeks following surgery. There were 52 patients who filled out a post-operative pain questionnaire at 4 weeks in the pre-emptive pain management group (Figure S1). In this subset of patients, the median pain score was 1.5 with IQR (0.0, 3.0) on a 1–10 pain scale.
Discussion
Our pre-emptive pain management program was associated with a significant reduction of opioid prescriptions after pulmonary resection. Patients who went home without opioids did not later require an outpatient opioid prescription, and the average self-reported pain score was very mild pain at a 4-week follow-up appointment. This result was likely due to success of the ERAS protocol—especially the pain management aspect of the protocol, wherein patients were given medication prior to experiencing pain rather than waiting to experience pain. This pre-emptive pain control sought to prevent central sensitization caused by the incisional injury and the subsequent inflammatory cascade (9). Our paradigm shift from giving hydromorphone PCA after surgery to giving around-the-clock, multimodal pain medication prior to making an incision made a dramatic difference in the management of pain after surgery. Long-acting local anesthetics, such as liposomal bupivacaine, also impacted patients’ experiences of pain after surgery when used effectively. By reducing patient pain, the combination of multimodal pain medication and a long-acting local anesthetic subsequently reduced the number of opioid prescriptions.
There is controversy, however, about the efficacy of liposomal bupivacaine in the management of pain after pulmonary resection. Certain studies show no significant difference in pain levels for patients using liposomal bupivacaine versus bupivacaine pain medication (10), while other studies show a significant difference in the opioid requirement after surgery (11) and the length of stay after minimally invasive thoracic surgical procedures (12). The different outcomes may be due to the difference in the technique used to inject the liposomal bupivacaine. We found the direct injection of the undiluted liposomal bupivacaine from the 2nd to the 10th intercostal nerves from inside the chest cavity at the beginning of the case provided the best nerve block after pulmonary resection. This injection, along with around-the-clock acetaminophen and gabapentin with or without an NSAID, made an impact in patients’ experiences with pain. Finally, once the chest tube was removed, most of the pain dissipated, making it easier for patients to be discharged home with a regimen of non-opioid pain medication.
To our surprise, chronic opioid pain medication use was not a factor in being discharged home with opioid pain medications. This is likely due to patients who are on chronic opioid pain medications were asked to have their opioid medications prescribed by their pain specialist to reduce duplicate opioid prescriptions. In our experience the patients who take chronic opioid pain medications quickly failed the non-opioid pain medication and required hydromorphone PCA to manage their post-operative pain. In addition, we found that the ERAS protocol alone did not have an impact on decreasing opioid prescriptions after surgery. This result has also been seen in other studies where the ERAS protocol alone did not decrease the number of opioid prescriptions at the time of discharge (13). This finding likely stems from the fact that providers are not very good at predicting the future opioid needs of the patients: studies have suggested that about 70% of the opioids prescribed after surgery are never used by patients (14-16). Our pre-emptive pain management program, however, successfully decreased the amount of opioid prescriptions by changing the default approach, shifting from an opioid prescription at discharge to a non-opioid pain medication after surgery. Finally, our study also found that the use of robot technology was associated with a decrease in opioid prescriptions after pulmonary resection when compared to an open or VATS procedure: using robot technology in pulmonary resection decreases the rate of thoracotomy compared to use of VATS technology (17), and patients with thoracotomy require opioids at discharge to manage post-operative pain.
As the opioid epidemic has made its way into the spotlight over the last several years, experts have worked together to develop different strategies to change opioid prescribing practices across the United States. For example, there have been efforts to minimize the number of unused opioid tablets prescribed by generating a consensus on the minimum number of tablets thought to be needed post-operatively for certain common surgical procedures (18). While these efforts aim to reduce unnecessary opioid prescriptions, our study highlights how we can manage the majority of patients after surgery without opioids as a discharge medication.
A major limitation of our study is the retrospective nature of the cohort study; however, the data was collected prospectively for the STS general thoracic surgery program, and the information about opioid prescriptions and the calls made to the clinic after surgery were obtained from medical records. Moreover, while the sample size in this study is small, it was designed to detect patterns in opioid prescriptions after the implementation of a non-opioid pain management program.
In conclusion, we found that our pre-emptive pain control program was associated with a decrease in opioid prescriptions after pulmonary surgery. We believe that with our pre-emptive pain control program, along with the ERAS program, we can significantly decrease not only opioid-naïve patients’ dependence on opioids after surgery, but also the availability of opioids in the community.
Acknowledgments
We thank Mark Celeste for language editing of this manuscript. We thank Kathryn J. Schulze and Debra Selig-Rosen for providing the data collected for the Society of Thoracic Surgeons at Houston Methodist Hospital.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-431
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-431). EYC reports personal fees from Veran Medical Technologies, outside the submitted work; MPK reports personal fees from Veran, personal fees from Intuitive Surgical, personal fees from Medtronic, outside the submitted work and he serves as an unpaid editorial board member of Journal of Thoracic Disease from Sep 2018 to Aug 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board of Houston Methodist Research Institute. The patient who were treated from 2012–2015 waived informed consent under Pro00013298. The patient who were treated from 2016–2018 signed informed consent for the study under Pro00013680.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Seth P, Rudd RA, Noonan RK, et al. Quantifying the Epidemic of Prescription Opioid Overdose Deaths. Am J Public Health 2018;108:500-2. [Crossref] [PubMed]
- Clarke H, Soneji N, Ko DT, et al. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ 2014;348:g1251. [Crossref] [PubMed]
- Alam A, Gomes T, Zheng H, et al. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med 2012;172:425-30. [Crossref] [PubMed]
- Moshfegh J, George SZ, Sun E. Risk and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients With Newly Diagnosed Musculoskeletal Pain in the Neck, Shoulder, Knee, or Low Back. Ann Intern Med 2019;170:504-5. [Crossref] [PubMed]
- Kim MP, Chan EY, Meisenbach LM, et al. Enhanced recovery after thoracic surgery reduces discharge on highly dependent narcotics. J Thorac Dis 2018;10:984-90. [Crossref] [PubMed]
- Kim MP, Chan EY. "Five on a dice" port placement for robot-assisted thoracoscopic right upper lobectomy using robotic stapler. J Thorac Dis 2017;9:5355-62. [Crossref] [PubMed]
- Wasserman L. Bayesian Model Selection and Model Averaging. J Math Psychol 2000;44:92-107. [Crossref] [PubMed]
- Dunson DB, Herring AH. Bayesian model selection and averaging in additive and proportional hazards models. Lifetime Data Anal 2005;11:213-32. [Crossref] [PubMed]
- Kissin I. Preemptive analgesia. Anesthesiology 2000;93:1138-43. [Crossref] [PubMed]
- Rincavage M, Hammond L, Reddy S, et al. Pain control using liposomal bupivacaine versus bupivacaine for robotic assisted thoracic surgery. Int J Clin Pharm 2019;41:258-63. [Crossref] [PubMed]
- Parascandola SA, Ibañez J, Keir G, et al. Liposomal bupivacaine versus bupivacaine/epinephrine after video-assisted thoracoscopic wedge resection†. Interact Cardiovasc Thorac Surg 2017;24:925-30. [Crossref] [PubMed]
- Dominguez DA, Ely S, Bach C, et al. Impact of intercostal nerve blocks using liposomal versus standard bupivacaine on length of stay in minimally invasive thoracic surgery patients. J Thorac Dis 2018;10:6873-9. [Crossref] [PubMed]
- Brandal D, Keller MS, Lee C, et al. Impact of Enhanced Recovery After Surgery and Opioid-Free Anesthesia on Opioid Prescriptions at Discharge From the Hospital: A Historical-Prospective Study. Anesth Analg 2017;125:1784-92. [Crossref] [PubMed]
- Feinberg AE, Chesney TR, Srikandarajah S, et al. Opioid Use After Discharge in Postoperative Patients: A Systematic Review. Ann Surg 2018;267:1056-62. [Crossref] [PubMed]
- Hill MV, McMahon ML, Stucke RS, et al. Wide Variation and Excessive Dosage of Opioid Prescriptions for Common General Surgical Procedures. Ann Surg 2017;265:709-14. [Crossref] [PubMed]
- Thiels CA, Anderson SS, Ubl DS, et al. Wide Variation and Overprescription of Opioids After Elective Surgery. Ann Surg 2017;266:564-73. [Crossref] [PubMed]
- Kim MP, Nguyen DT, Meisenbach LM, et al. Da Vinci Xi robot decreases the number of thoracotomy cases in pulmonary resection. J Thorac Dis 2019;11:145-53. [Crossref] [PubMed]
- Overton HN, Hanna MN, Bruhn WE, et al. Opioid-Prescribing Guidelines for Common Surgical Procedures: An Expert Panel Consensus. J Am Coll Surg 2018;227:411-8. [Crossref] [PubMed]