
Tumor infiltrating T cells influence prognosis in stage I–III non-small cell lung cancer
Introduction
Worldwide, lung cancer harbors paramount position in cancer specific incidence and still is the leading cause of cancer related mortality (1). Despite awareness campaigns, relative incidence ratios of lung cancer in young women resident in western countries are rising (2). With respect to histology, two major subtypes must be distinguished. Whereas non-small cell lung cancer (NSCLC) comprises about 85% of all new-diagnosed cases, small cell lung cancer (SCLC) is the less common (~14%) but more aggressive subtype (3). By genotyping (4) and sequencing oncogene and tumor suppressor gene loci (5), the latter entity of SCLC is nowadays assorted with large cell neuroendocrine carcinomas (LCNEC), typical carcinoids and atypical carcinoids (5-7). Albeit the reclassification of large cell carcinoma (LCC), two distinct histopathological entities represent classical NSCLC subtypes (8,9), i.e., adenocarcinoma (ADC) of the lung and squamous cell carcinoma (SCC) of the lung.
The arise of immunotherapies (10) brought forth a better understanding of lung cancer tumor infiltrating leukocytes (TILs) (11-13). Focusing on therapeutic implications, leukocyte levels and activation markers in lung cancer patients’ blood (14,15) have been investigated. In addition to invading macrophages (12,13), tumor infiltrating T cells are the focus of current research efforts (16,17).
T cells are lymphatic cells that are primarily characterized by expression of the T cell receptor (TCR) and cluster of differentiation 3 (CD3), the T cell co-receptor. As a result of the interaction of the TCR complex with the major histocompatibility complex II (MHC II) of antigen presenting cells, an avalanche maturation and activation process from naïve T cells to effector or memory T cells is initiated. Among T cells, three major subtypes must be distinguished: besides cytotoxic CD3+ CD8+ T cells (Tc), there are two types of CD3+ CD4+ T cells (18). Polypotent CD4+ T cells develop into regulatory CD4+ CD25+ T cells (Treg) and into conventional CD4+ T helper (Th) cells, containing three subtypes (Th1, Th2 and Th17) (19,20). While mature Th1 cells activate macrophages and CD8+ Tc cells via interferon γ (IFNγ) and thereby direct immune response against intracellular pathogens and autoimmunity, Th2 cells mediate defense mechanisms against extracellular pathogens such as helminths by activating eosinophils, basophils and humoral responding B cells via interleukin 4 (IL4) (18). Finally, Th17 cells maintain the immune response against extracellular fungal or bacterial infections by activation of neutrophils via IL17 (20).
With regard to CD4+ and CD8+ T cells, a higher T lymphocytic infiltration in NSCLC is considered a favorable prognostic marker (13,16,17,21). Studies focused on the interaction of distinct T cell infiltration with epithelial to mesenchymal transition (EMT) in NSCLC: a more ‘mesenchymal state’, predicted by a sixteen-gene signature, was associated with few CD4+ cells in ADC and reduced CD4+ and CD8+ cell counts in SCC (22).
Other than CD4+ Th cells, CD25+ co-expressing Treg cells occur within peripheral blood, healthy tissues and malignant tissues. Most relevantly, these cells express Forkhead box transcription factor (FOXP3) (23). Of interest, FOXP3 is not exclusively expressed on Treg cells but might also be transiently found on activated CD4+ CD25– Th cells (24,25) and CD8+ Tc cells (26). A closer characterization of Treg cells allows the division into suppressive Treg cells, expressing the cytotoxic T lymphocyte associated protein 4 (CTLA4), and resting Treg cells that lack CTLA4 (19,23). Treg cells control self-tolerance via cell-cell-interaction and, amongst others, secretion of transforming growth factor β (TGFβ). FOXP3 supports the Treg cell function by mediating downregulation of both T cell expansion and inflammatory cytokine overexpression (27). Hence, high levels of Treg cells proved beneficial in controlling autoimmune disorders whereas depletion of Treg cells is considered to enhance immune response against tumor tissue (20). However, TGFβ itself was found to be a driver of EMT (28), a process that promotes malignancy and metastatic conversion by tumoral loss of cell-cell-junctions and a gain in motility (29). In autoimmune diseases, the suppressive function of thymic Treg cells and circulating Treg cells is well characterized, whereas the role of a Treg cell phenotype detected in tumor microenvironment is ambiguous. Here, tumor infiltrating Treg cells might be substantially different from circulating Treg cells (23). There is evidence that tumor Treg cells share similarity of surface stimulatory and inhibitory receptors with cytotoxic effector T cells (23). Using single cell analysis, De Simone et al. revealed that NSCLC tumoral Treg cells exhibited a tumor specific surface signature, including overexpression of e.g., non-tumoral PD-L1. Here, Treg surface expression levels of PD-L1 were significantly lower when taken from both blood and healthy lung tissue (30). Subsequent to tumor endothelial VEGF secretion, Treg cells migrate into tumor tissue (31,32). Here they ought to orchestrate the immune response by inhibiting antigen presenting cells and cytotoxic T cells via secretion of IL10 and TGFβ as well as by withdrawal of IL2 (27).
Both in colorectal cancer (32) and in head and neck SCC (33), high infiltration rates of Treg cells were associated with improved prognosis. In hepatocellular carcinoma however, the effect was vice versa (32). In NSCLC, Treg infiltration has been analyzed and discussed very controversially (13). While Kayser et al. demonstrated that high levels of CD4+ CD25+ Treg cells were associated with improved survival in patients suffering from ADC and SCC of all stages (34), Kinoshita et al. reported unfavorable outcomes in stage I ADC patients when diagnosed high levels of FOXP3+ Treg infiltration (35). Moreover, stage IV epidermal growth factor receptor (EGFR) mutant ADC patients, pretreated with EGFR-monoclonal antibody and nivolumab, benefited of high CD4+ and FOXP3+ T cellular infiltration (36). In contrast, other studies reported on high FOXP3+ T cell infiltration to predict poor survival in NSCLC (37-39).
Taking into account the contrary results of FOXP3 positivity in activated CD4+ CD25– Th and CD8+ Tc cells (26,40,41), the influence of FOXP3+ Treg cells in lung cancer progression has not fully been clarified. Cofactors, such as tumor stage, histology and distinct tumor biology might influence the regulatory effect. To investigate the prognostic impact of Th cells, Tc cells and Treg cells in stage I–III NSCLC, we performed the underlying, retrospective immunohistochemical analysis with focus on specific markers such as CD4, CD8 and FOXP3.
Methods
Study collective
Retrospectively, we analyzed n=294 cases of NSCLC patients, who were surgically treated at the Thoracic Surgery Department of the St. Georg’s Clinic Ostercappeln between December 1998 and November 2004. In consequence of the therapeutic dates, the sixth edition of tumor nodule metastasis (TNM) system proposed by the Union Internationale Contre le Cancer (UICC) (42) was applied. An update towards later TNM staging system editions was not possible due to ethical concerns regarding privacy and data protection.
Tissue preparation
We analyzed the surgically resected primary tumor tissue samples by using 4-µm-thick formalin-fixed paraffin-embedded (FFPE) tissue microarrays (TMA). Three punch cores from the original tumor specimen solely represented one single NSCLC case in the present cohort (43). Tissue preparation was performed according to the suggestions of von Wasielewski et al. (44). In detail, the hematoxylin and eosin stained primary tumor slides were used to identify representative and non-necrotic tumor plots including both, vital tumor cells as well as stromal cells and infiltrates. Moreover, three punch cores ought to dissociate at least 1 mm within the primary tumor block. These areas were then marked on the paraffin embedded tumor block and punched out with a 0.6 mm biopsy needle. Afterwards, gained tissue was melted within in the accepting paraffin block.
Immunohistochemistry
We used the peroxidase-conjugated avidin-biotin method to perform immunohistochemistry. Antibodies included rabbit CD4 monoclonal primary antibody (Roche/Ventana clone SP35; Catalog Number 790-4423), rabbit CD8 monoclonal primary antibody (Roche/Ventana clone SP57; Catalog Number 790-4460) and rabbit FOXP3 polyclonal primary antibody (Abcam clone ab4728; Catalog Number ab4728). In sum, TMA sections were deparaffinized in xylene and rehydrated through graduated ethanol-solutions at ambient temperature. We incubated the primary antibodies for 30 minutes at room temperature. After washing, the incubation of the paraffin sections with biotinylated secondary antibodies was performed. Using 3-amino-9-ethylcarbazole as a substrate, immunoreactions were detected (Roche/Ventana Optiview DAB IHC detection KIT; Catalog Number 760-700). Positive controls were performed on human tonsil tissues (Figure S1).
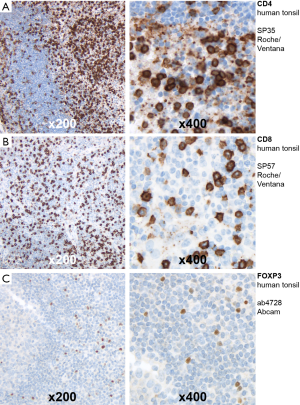
Tissue analysis
Tissue was analyzed at a Leica DM4B and an Olympus BX51 microscope with an average magnification of ×200 by ABS, LHS and BH. Deviations in the microscopic analysis were discussed interdisciplinary. To detect distinct staining in FOXP3 stained tissue, an intermittent magnification of ×400 was allowed. The TIL infiltration grade was studied in accordance to the method proposed by the International TILs Working Group 2014 (45). Hence, TIL infiltration was documented as percent of the cells on one punch core. Subsequently, CD4+, CD8+ and FOXP3+ cells were then assessed as percent of TILs. As proposed by the International TILs Working Group 2014, the analyzed TILs derived from stromal areas and excluded granulocytes and macrophages in necrotic or inflammatory sections (45). Additionally, highly infiltrated areas by granulocytes or macrophages were visually excluded from the analysis. As each NSCLC case was represented by three punch cores, each evaluated case accommodated a maximum of three independent TIL counts and three independent counts of CD4+, CD8+ and FOXP3+ cells, respectively. Data was later on harmonized to a mean and median TIL count as well as mean and median CD4+, CD8+ and FOXP3+ cell count, each dependent and independent from TIL infiltration grade.
Post-hoc PD-L1 correlations
With regard to Schmidt et al. (46), PD-L1 stains were performed on this cohort beforehand via rabbit PD-L1 monoclonal primary antibody (Cell Signaling Technology clone E1L3N, Catalog Number 13684). Herein, n=293 cases could be matched to perform post-hoc correlative analyses of PD-L1 and TIL infiltration. As described by Schmidt et al., tumors were evaluated PD-L1 positive, if at least 5% of tumoral tissue was stained positively. Moreover, staining intensity was semi-quantitatively documented via the immunoreactive score (IRS) (i.e., 0= no staining, 1= mild staining, 2= moderate staining, 3= intense staining) (47). To simplify analysis, IRS 0–1 tissue was deduced ‘PD-L1 negative’ and IRS 2–3 tissue ‘PD-L1 positive’.
Statistical analysis
Mean, standard deviation (SD), median, interquartile range (IQR; Q1–Q3) as well as raw count and frequencies were used to describe the patient cohort. Twofold associations between categorical variables were analyzed via Fisher’s exact test or Chi-square test, if applicable. Continuous and ordinal variables were tested using either unpaired t-test or Mann-Whitney-U test depending on the normality of the data. Associations of more than two groups (e.g., stage variable) with categorical and continuous outcomes were analyzed using Chi-square test, Kruskal-Wallis test or via one-way-ANOVA, in case of continuous and normally distributed values. The area under the receiver operating characteristic curve (ROC) was used to approximate cutoff values for CD4+ and CD8+ cells via Youden Index for binary classification split by median overall survival (OS).
OS was defined as time (days) between histopathological diagnosis and death or censoring. Progression-free survival (PFS) was defined as time (days) between histopathological diagnosis and first relapse, progress after initial treatment, death or censoring, depending on the first chronological appearance. Univariate survival analyses compared OS and PFS between groups by using log-rank tests. Kaplan-Meier plots then were used to visualize survival differences. Multivariate survival analyses were performed via Cox proportional hazards model by applying likelihood ratio tests with a forward variable selection. Inclusion criterion was set to 0.05. Hazard ratios (HR) are presented with 95% confidence intervals (95% CI). Data collection as well as calculations were performed using IBM® SPSS® Statistics Version 25 (released 2017, IBM Corp., Armonk, NY, USA). Graphic data output was partly generated in Microsoft Office Professional Plus 2010 Excel (released 2010, Microsoft Corp., Redmond, WA, USA). The local significance level was set to 0.05. Due to the explorative character of the analysis, an adjustment to multiplicity was not determined.
Results
Baseline characteristics
Baseline data of the investigated study cohort of n=294 NSCLC patients with stage I–III disease is summarized in Table 1. Briefly, 80% of the patients were male. Mean age at time of diagnosis was 65.3 years. While 93% of the patients had a good performance (ECOG 0–I), 7% revealed limited performance status (ECOG II–III) previous to NSCLC diagnosis. 77% of the patients were smokers and the mean relative forced expiratory volume in 1 second (FEV1) was 80.8% (±20.1%). Regarding tumor histology, 48% exhibited SCC morphology, 38% were ADC and 14% LCC. Furthermore, one third of the tumors had a low tumor grade (G1 and G2), whilst two thirds had high tumor grades (G3 and G4). Following the UICC 6th edition TNM proposal, 53% of the patients had stage I disease, 25% had stage II tumors and 22% stage III tumors. More specifically, lymphonodal spread (pN+) was found in 39% of the cases. While 70% of the patients did not receive any neoadjuvant treatment, 30% were treated by radiotherapy or chemotherapy before surgical treatment and acquisition of tumor specimen. PD-L1 stain was negative in three fourth of the specimen (IRS 0–1). The mean TIL count was 26.7%. Of these, mean and median amounts of CD4+ cells were 22% and 20%, of CD8+ cells 24% and 20% and of FOXP3+ cells 2% and 0%, respectively. In terms of survival, median PFS was 932 [95% confidence interval (CI), 691–1,173] days, median OS was 1,316 (95% CI, 986–1,646) days, and median follow up was 2,686 (95% CI, 2,557–2,815) days, respectively.
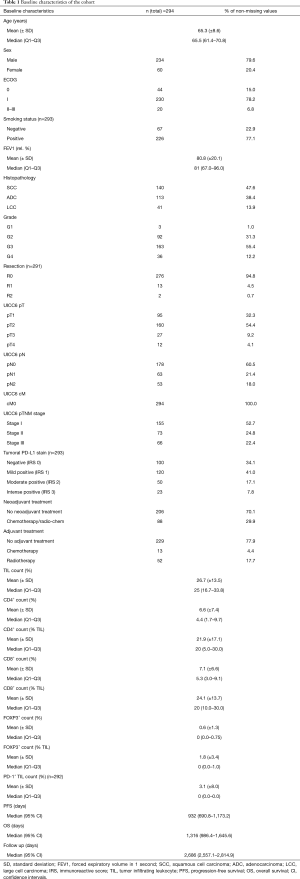
Full table
TIL associations with histomorphological and clinicopathological features
Figure 1 exemplary depicts the implemented CD4 (Figure 1A,C,E) and CD8 (Figure 1B,D,F) stains on matching cores. Whereas Figure 1A,B represent lymphocytic stains in stroma of a well differentiated tumor, Figure 1C,D reveal a predominant CD4+ TIL infiltration on a stromal column, whereas CD8+ TILs can be found in tumor area as well as in the column of stroma. Figure 1G,H depict negative and positive FOXP3 TIL stains.
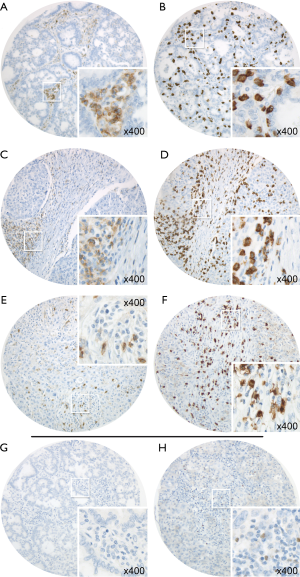
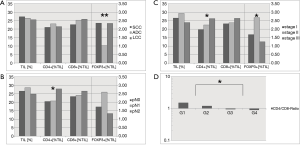
Figure 2 reveals associations of TIL-infiltration in general and CD4+, CD8+ and FOXP3+ T lymphocytic infiltration with tumor-biologic markers such as histology, lymphonodal spread, TNM-stage and tumor grading. Interestingly, FOXP3+ T cells appeared less frequent in ADC (mean infiltration 1.03%) than in SCC (2.35%) or LCC (2.34%) (P=0.006). No significant difference between morphologic entities ADC, SCC and LCC was found for percentage TIL count, CD4+ or CD8+ count (Figure 2A). In terms of CD4/CD8 ratio, CD4 dominance (ratio >1) was associated with low grade tumors, and CD8 dominance (ratio <1) with high grade tumors (P=0.020) (Figure 2D). While the pT stage 1–4 was not associated with higher levels of TILs, CD4+ T cells, CD8+ T cells or FOXP3+ T cells (all P>0.05, data not shown), CD4+ cells were more present in pN2 tumors (28.0% CD4+ TILs) than in pN0 (20.5%) or pN1 (20.8%) cases (P=0.014). No such associations were found for CD8 or FOXP3, respectively (all P>0.05) (Figure 2B). As a consequence of the pN stage, there was an increase in CD4 levels in TILs at higher tumor stages (stage I 19.8%, stage II 22.6%, stage III 26.2%, P=0.034). Here, FOXP3 density was associated with stage II tumors (P=0.036), too (Figure 2C). Associations of TIL count, CD4+ TIL count, CD8+ TIL count or FOXP3+ TIL count were neither found for age (<65, ≥65 years), sex (male, female), ECOG (0, 1, 2, 3), smoking status (non-smoker, smoker) nor neoadjuvant treatment (no neoadjuvant treatment, neoadjuvant treatment) (all P>0.05).
Classification and characteristics of CD4+, CD8+ and FOXP3+ expression split subcohorts
In terms of prognostic impact of CD4+ and CD8+ T lymphocyte infiltration in tumor tissue, we performed an area under the receiver operating characteristic curve analysis via Youden index for median OS. Highest sensitivity and specificity was bordering 20% cutoff value for CD4+ and CD8+ cells, respectively. Hence, we compared low infiltration rates (i.e., <20% of TILs) with high infiltration rates (i.e., ≥20% of TILs). For FOXP3+ cells, we compared specimen absent of FOXP3+ Treg cells (i.e., 0% of TILs) with FOXP3+ detected cells in tumor and stromal tissue (i.e., ≥1% of TILs). Subsequently, the deriving subcohorts were analyzed; see Table S1 and Figure 2. Here, n=161 patients revealed low CD4+ infiltration rates, whereas n=133 patients had a high CD4+ lymphocytic infiltration. With respect to age, gender, smoking behavior, histopathology, tumor grading and pT stage, no significant difference in high and low CD4+ infiltrated tumor tissue specimen was detected (all P>0.05). In line with the raw TIL counts, high CD4+ lymphocyte infiltration predicted pN2 tumors (P=0.039) and higher pTNM stages (P=0.047), see also Figure 2B,C. Neither for high (n=155) or low (n=139) CD8+ infiltrated tumors, nor for tumors with (n=123) or without FOXP3+ infiltrating cells (n=171) differences in age, gender distribution, smoking status, histopathology, tumor grading, pT, pN or pTNM staging any differences in the derived subcohort were found (all P>0.05). As a consequence of the proposed subcohorts for CD4+ and CD8+ dichotomy, mean TIL count was significantly lower in the low CD4+ (P=0.002) and low CD8+ subgroup (P<0.001), see Table S1.
Full table
Univariate OS and PFS analysis
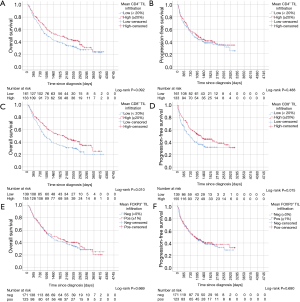
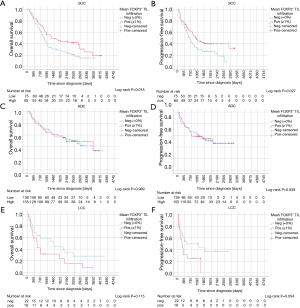
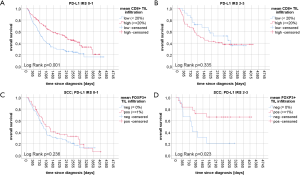
Kaplan-Meier curves of univariate survival analyses regarding CD4+, CD8+ and FOXP3+ TIL infiltration can be found in Figure 3. Univariate survival analyses yielded improved prognostic outcome of tissue with high CD8+ TIL levels (P=0.01 for OS, P=0.015 for PFS, Figure 3C,D). Neither the presence of FOXP3+ cells (Figure 3E,F) nor high CD4+ infiltration levels (Figure 3A,B) resulted in improved OS or PFS, univariately (all P>0.05). However, when focusing on SCC patients solely, FOXP3+ Treg cells were associated with improved PFS (P=0.027) and OS (P=0.018) (Figure 4A,B). However, in ADC and LCC no association of FOXP3+ Treg cells with survival was found (Figure 4C,D,E,F). Despite specific T cell subsets, TIL density itself did not affect PFS or OS (all P>0.05, data not shown).
Multivariate OS and PFS analysis
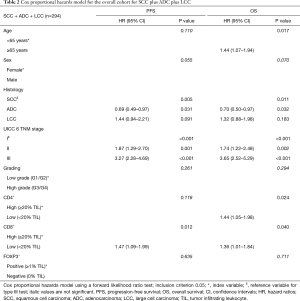
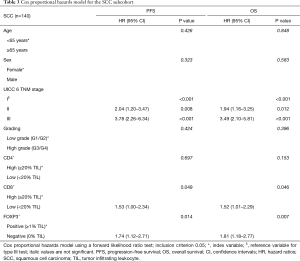
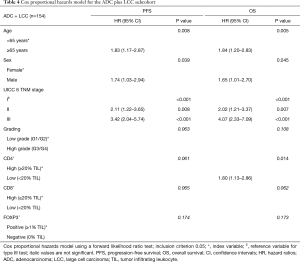
Multivariate prognostic analyses for OS and PFS were performed for the whole cohort (n=294, Table 2) as well as for SCC (n=140, Table 3) and ADC plus LCC subcohorts (n=154, Table 4), respectively. While analyzing the whole cohort, we used histologic growth pattern (SCC vs. ADC vs. LCC) as an additional independent factor for the analysis. By multivariate survival analysis of the whole cohort as well as of SCC and ADC plus LCC subgroups we evaluated the independent factors of age (≤65 vs. ≥65 years), sex (female vs. male), UICC pTNM 6th edition stage (stage I vs. stage II vs. stage III), tumor grading (G1/2 vs. G3/4), CD4+ (≥20% TILs vs. <20% TILs), CD8+ (≥20% TILs vs. <20% TILs) and FOXP3+ (≥1% TILs vs. 0% TILs). In the full cohort (Table 2), next to age, histology and TNM stage, a low CD4+ TIL infiltration (<20% of TILs: HR: 1.44, P=0.024) as well as CD8+ TIL infiltration (<20% of TILs: HR: 1.36, P=0.04) were predictors of dismal OS. For PFS, only histology, TNM stage and CD8+ infiltration were independent prognostic factors, respectively. However, focusing on the SCC subgroup (Table 3), apart from the TNM stage, only low CD8+ TIL infiltration (HR: 1.52 for OS, HR: 1.53 for PFS, all P<0.05) and negative FOXP3+ Treg infiltration (HR: 1.81 for OS, HR: 1.74 for PFS, all P<0.02) were independent predictors of dismal outcome. Yet, in ADC and LCC subcohort (Table 4), only CD4+ infiltration (<20% of TILs: HR: 1.80, P<0.02) sustained an independent prognostic factor for OS, along with age, sex and TNM stage.
Full table
Full table
Full table
Post-hoc PD-L1 correlations and survival analysis
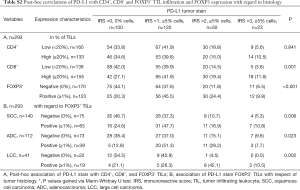
We performed post-hoc PD-L1 tumor stain correlations (46) with CD4+, CD8+ and FOXP3+ tumor infiltrating lymphocytes. Raw and percentage counts of the evaluations can be found in Table S2. The infiltration ratio of CD4+ lymphocytes was not associated with PD-L1 negativity or positivity, irrespective of the tumors histology (SCC P=0.835, ADC P=1.000, LCC P=0.734, data not shown). Interestingly, high CD8+ infiltration was correlated with PD-L1 positive tumors (P=0.001). Likewise, the presence of FOXP3+ Treg cells was significantly more likely in PD-L1 positive NSCLC tissues (P<0.001), irrespective of the underlying histology (SCC P=0.006, ADC P=0.023, LCC P=0.002, see Table S2). Univariate OS analyses split by low and high CD8+ lymphocytic infiltration resulted in beneficial survival for high CD8+ TILs only in PD-L1 low tissue (i.e., IRS 0–1, P=0.001) but not in PD-L1 high tissue (i.e., IRS 2–3, P=0.335) in the overall cohort. On the contrary, in SCC, FOXP3+ Treg cells now resulted in improved survival only in PD-L1 overexpressing tissue (i.e., IRS 2–3, P=0.023) but not in PD-L1 low tumors (i.e., IRS 0–1, P=0.263). Corresponding Kaplan-Meier curves can be found in the Figure S2.
Full table
Discussion
Among lymphocytic infiltration in NSCLC tissues, T cells dominate (14). With regard to checkpoint inhibition, CD8+ T cells in particular are in the scientific focus since anti-tumoral activity of cytotoxic T cells can be interfered by PD-1/PD-L1 axis of tumoral self-tolerance (10). Here, we depicted the influence of T cells, characterized by the expression of CD4, CD8 and FOXP3, in NSCLC tumor microenvironment. Basically, the underlying analysis was carried out to obtain clinicopathologic and prognostic information with focus on T cellular infiltration in NSCLC. However, the presented data derives retrospective analyses. Moreover, we did not adjust p-values to multiple testing. Therefore, the presented statements may only be used to generate or prove hypotheses.
As revealed by flow cytometry in a smaller cohort (14), we support the thesis, that histology does not affect CD4+ or CD8+ T cell infiltration (Figure 2A). While CD8+ T cells were neither associated with primary tumors’ size (pT), nodal status (pN) nor with pTNM stage (Figure 2A,B,C), a higher CD4+ TIL density correlated with the event of lymphonodal spread (N2) (Figure 2B) and higher pTNM tumor stages (Figure 2C). In terms of tumor grading, however, the CD4/CD8 ratio was shifted in favor of CD8 (<1) for high-grade tumors (G3/4) (Figure 2D). With respect to tumoral PD-L1 stain, we were able to demonstrate, that the beneficial prognostic effect of tumor infiltrating CD8+ lymphocytes does not account for tumors with high PD-L1 expression (Figure 2A,B).
EMT is a crucial pathway in cancerogenesis affecting invasiveness of cancer cells by promoting motility and trans-endothelial migration. This process can be studied by analyzing the tumor for ‘mesenchymal’ markers such as N-cadherin (48) and vimentin (49). Both factors are overexpressed in high grade NSCLC (G3/4) indicating that dedifferentiation of the tumor from its cell origin might result from EMT. In this context, our results support the idea, that tumor grading is associated with EMT, since the orchestration of Th, Treg and Tc cellular immunosurveillance in NSCLC is directly linked to EMT. Of interest, in ADC decreasing fold change of Treg cells in the tumor microenvironment was found in a higher ‘mesenchymal state’ in EMT, while in SCC Treg cell depletion has not been described in the process of EMT (22). We were able to reproduce these findings, by documenting reduced FOXP3+ infiltration in ADC but not in SCC or LCC, see Figure 2A. Still, the number of T cells in the tumor did not change in EMT, but activated CD4+ Th cells were especially prominent in ‘epithelial state’ tumors in both ADC and SCC (22). This correlates with the grade-dependent CD4/CD8 ratio finding in Figure 2D. However, for pancreatic ADC cells (50), IFNγ, eventually produced by CD4+ Th1 cells, was shown to induce EMT and the overexpression of PD-L1 on tumor tissue. And yet in NSCLC, both ADC and SCC presented significantly higher levels of TGFβ1, IL2, IL10 and IFNγ in a ‘mesenchymal state’ compared to the ‘epithelial state’ (22). Alas in this NSCLC cohort, CD4+ lymphocytic infiltration neither deduced to PD-L1 positive SCC, ADC nor LCC (all P>0.05, data not shown).
Interestingly, Vahl et al. detected for ADC that the ‘immunosuppressive’ IL10, inter alia produced by M2 macrophages, activated Treg cells and lead to both tumoral and FOXP3+ Treg cellular upregulation of the IL10 receptor (IL10-R). By negative feedback via the Treg cells, this finally resulted in less PD-L1 expression in ADC and contributed to a milieu enriched by pro-tumor IL10, as well as the presence of M2 macrophages and PD1+ Th exhausted cells. However, in SCC the presence of IL10 resulted in tumoral but not in Treg cell upregulation of IL10-R. Likewise, IL10-R upregulation correlated with higher levels of tumoral PD-L1 (51). Alas, anti-tumoral implications of Treg cell presence in SCC were not further elucidated. Unlinke Vahl et al., Giatromanolaki et al. and Silva et al. showed, that high FOXP3 infiltration levels were associated with high PD-L1 stain, irrespective of histology (21,52). Our findings enqueue to the positive association of PD-L1 expressing tumors with a higher prevalence of FOXP3+ Treg cell infiltrates, irrespective of tumor histology (SCC P=0.006, ADC P=0.023, LCC P=0.002).
With respect to prognosis, CD4+ TIL infiltration, prominent in ‘epithelial state’ NSCLC (22), was associated with an improved OS in our multivariate analysis (Table 2). In addition, CD4+ TIL infiltration affected OS in the ADC plus LCC cohort (Table 4), while CD4+ TIL infiltration for SCC had no prognostic impact (Table 3). For SCC, Sterlacci et al. stated a high CD4/CD8 (≥1) ratio to be associated with inferior outcome (53). Alas, we could not confirm these findings in our study cohort (Figure S3). Next, we identified strong CD8+ TIL infiltration to be a marker of improved prognosis (16,17,54) in the entire cohort both for OS (Figure 3C, Table 2) and for PFS (Figure 3D, Table 2). Both Lin et al. and Kinoshita et al. described high CD8+ TIL infiltration as beneficial in ADC (55,56) and non-ADC (56). However, in our analyses, a high CD8+ TIL infiltration in SCC but not in ADC plus LCC was found to be an independent prognostic factor in stage I–III NSCLC (Tables 3,4).
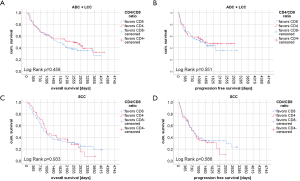
Regarding FOXP3+ Treg cellular infiltration, our results contribute to the findings of other publications (11,37). Similar to our data, these authors reported less FOXP3+ TIL cells in non-squamous NSCLC than in SCC. Here, we identified that specifically ADC but not LCC lack FOXP3+ T-lymphocytic cells (Figure 2A). Moreover, our data indicate that increased evidence of stromal FOXP3+ cells resulted in improved survival in SCC (11) (Table 3 and Figure 4A,B), especially in PD-L1 positive tissue (Figure S2D), but not in ADC (Figure 4C,D) or LCC (Figure 4E,F). At first glance the results for the SCC subcohort are contradictory since many retrospective survival analyses on NSCLC depicted high Treg cell infiltration to be an unfavorable prognostic factor (37-39). The latter findings were reproduced in cell culture: Here, presence of FOXP3+ Treg cells finally enhanced tumor growth and reduced apoptosis in 95D lung cancer cells (57). At the same time, however, in presence of FOXP3+ Treg cells local tumor cell migration was reduced and tumor cell adhesiveness was promoted (57). Hence, the effect of FOXP3+ Treg cells on NSCLC tissue is manifold and this cell-interaction especially in the light of tumoral PD-L1 expression needs further investigation. Still, we have to consider, that FOXP3 might transiently be expressed by conventional CD4+ CD25- Th cells or even cytotoxic CD8+ Tc cells (24-26,40,41). Therefore, the evaluation of tumoral Treg cells becomes even more challenging and might partly explain opposing prognostic evaluations on Treg cells in NSCLC.
With focus on the pathogenetic and prognostic impact of FOXP3+ Treg cells there is a paradoxon in solid tumors, outlined by Shang et al. in their meta-analysis (58). While in colorectal cancer (59), in head and neck SCC (33) as well as in esophageal cancer (58) a high proportion of FOXP3+ Treg cells resulted in superior OS, it was found reversely for breast cancer, hepatocellular cancer, pancreatic cancer, melanomas, ovarian cancer, renal cell carcinomas and cervical cancer (58). Nevertheless, for follicular lymphoma and Hodgkin’s lymphoma, FOXP3+ T cell infiltration was also associated with improved outcome (58). Shang et al. propose a prognostically beneficial role of FOXP3+ Treg cells in heavily granulocyte and macrophage infiltrated solid tumors with elevated amounts of cytokines (58). Of interest, in our study cohort TIL infiltration was non significantly higher in SCC compared to ADC but FOXP3+ infiltrates were more prominent in SCC and LCC than in ADC (Figure 2A).
In conclusion, unlike other publications (37-39), the present cohort did not reveal any association for high FOXP3+ Treg cell infiltration with dismal outcome in stage I–III tumors and the prognostic benefit of hig FOXP3+ infiltrates in PD-L1 positive SCC needs futher clarification. Nevertheless, high levels of CD8+ cells in the tumor microenvironment indicated improved OS and PFS in the overall cohort and SCC subcohort, especially if PD-L1 negative, while a high CD4+ TIL infiltration was associated with improved OS in the overall cohort as well as in the ADC and LCC subcohort. Yet, a CD8+ dominant T cell pattern was associated with high grade NSCLC tumors. The opposing results of TIL influence on SCC and ADC emphasize the need for further studies of T cell impact on NSCLC, especially against the background of histology, EMT state, PD-L1 expression and molecular biology.
Acknowledgments
We thank Inka Buchroth, Lydia Fälker and Christin Fehmer from the Gerhard Domagk Institute of Pathology, University Hospital Muenster for their excellent work on performing immunohistochemistry.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-3414a). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was performed in concordance with the ethical approval obtained from the concerned Ethical Committee in Münster (Az 2016-445-f-S and Reg.Nr.: 4XMüller1).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Fidler-Benaoudia MM, Torre LA, Bray F, et al. Lung cancer incidence in young women vs. young men: a systematic analysis in 40 countries. Int J Cancer 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Travis WD, Brambilla E, Müller-Hermelink HK, et al. editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press, 2004.
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. [Crossref] [PubMed]
- Rossi G, Bertero L, Marchiò C, et al. Molecular alterations of neuroendocrine tumours of the lung. Histopathology 2018;72:142-52. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Schulze AB, Evers G, Kerkhoff A, et al. Future Options of Molecular-Targeted Therapy in Small Cell Lung Cancer. Cancers (Basel) 2019;11:690. [Crossref] [PubMed]
- Shoshan-Barmatz V, Bishitz Y, Paul A, et al. A molecular signature of lung cancer: potential biomarkers for adenocarcinoma and squamous cell carcinoma. Oncotarget 2017;8:105492-509. [Crossref] [PubMed]
- Fisseler-Eckhoff A. Prognostic factors in histopathology of lung cancer. Front Radiat Ther Oncol 2010;42:1-14. [PubMed]
- Schulze AB, Schmidt LH. PD-1 targeted Immunotherapy as first-line therapy for advanced non-small-cell lung cancer patients. J Thorac Dis 2017;9:E384-6. [Crossref] [PubMed]
- Meng X, Gao Y, Yang L, et al. Immune microenvironment differences between squamous and non-squamous non-small-cell lung cancer and their influence on the prognosis. Clin Lung Cancer 2019;20:48-58. [Crossref] [PubMed]
- Rakaee M, Busund LR, Jamaly S, et al. Prognostic value of macrophage phenotypes in resectable non-small cell lung cancer assessed by multiplex immunohistochemistry. Neoplasia 2019;21:282-93. [Crossref] [PubMed]
- Soo RA, Teng RSY, Tai BC, et al. Prognostic significance of immune cells in non-small cell lung cancer: meta-analysis. Oncotarget 2018;9:24801-20. [Crossref] [PubMed]
- Stankovic B, Bjørhovde HAK, Skarshaug R, et al. Immune cell composition in human non-small cell lung cancer. Front Immunol 2019;9:3101. [Crossref] [PubMed]
- Manjarrez-Orduño N, Menard LC, Kansal S, et al. Circulating T cell subpopulations correlate with immune responses at the tumor site and clinical response to PD1 inhibition in non-small cell lung cancer. Front Immunol 2018;9:1613. [Crossref] [PubMed]
- Al-Shibli KI, Donnem T, Al-Saad S, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 2008;14:5220-7. [Crossref] [PubMed]
- Miotto D, Cascio N, Lo , Stendardo M, et al. CD8+ T cells expressing IL-10 are associated with a favourable prognosis in lung cancer. Lung Cancer 2010;69:355-60. [Crossref] [PubMed]
- Kanduri K, Tripathi S, Larjo A, et al. Identification of global regulators of T-helper cell lineage specification. Genome Med 2015;7:122. [Crossref] [PubMed]
- Guo X, Zhang Y, Zheng L, et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med 2018;24:978-85. [Crossref] [PubMed]
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood 2008;112:1557-69. [Crossref] [PubMed]
- Giatromanolaki A, Banham AH, Harris AL, et al. FOXP3 infiltrating lymphocyte density and PD-L1 expression in operable non-small cell lung carcinoma. Exp Lung Res 2019;45:76-83. [Crossref] [PubMed]
- Chae YK, Chang S, Ko T, et al. Epithelial-mesenchymal transition (EMT) signature is inversely associated with T-cell infiltration in non-small cell lung cancer (NSCLC). Sci Rep 2018;8:2918. [Crossref] [PubMed]
- Toker A, Ohashi PS. Expression of costimulatory and inhibitory receptors in FoxP3+ regulatory T cells within the tumor microenvironment: Implications for combination immunotherapy approaches. Adv Cancer Res 2019;144:193-261. [Crossref] [PubMed]
- Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3− T cells by T-cell receptor stimulation is transforming growth factor-β–dependent but does not confer a regulatory phenotype. Blood 2007;110:2983-90. [Crossref] [PubMed]
- Wang J, Ioan-Facsinay A, van der Voort EIH, et al. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol 2007;37:129-38. [Crossref] [PubMed]
- Pillai V, Ortega SB, Wang CK, et al. Transient regulatory T-cells: a state attained by all activated human T-cells. Clin Immunol 2007;123:18-29. [Crossref] [PubMed]
- Alissafi T, Hatzioannou A, Legaki AI, et al. Balancing cancer immunotherapy and immune-related adverse events: the emerging role of regulatory T cells. J Autoimmun 2019;104:102310. [Crossref] [PubMed]
- Gordian E, Welsh EA, Gimbrone N, et al. Transforming growth factor β-induced epithelial-to-mesenchymal signature predicts metastasis-free survival in non-small cell lung cancer. Oncotarget 2019;10:810-24. [Crossref] [PubMed]
- Antony J, Thiery JP, Huang RYJ. Epithelial-to-mesenchymal transition: lessons from development, insights into cancer and the potential of EMT-subtype based therapeutic intervention. Phys Biol 2019;16:041004. [Crossref] [PubMed]
- De Simone M, Arrigoni A, Rossetti G, et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity 2016;45:1135-47. [Crossref] [PubMed]
- Hansen W, Hutzler M, Abel S, et al. Neuropilin 1 deficiency on CD4 + Foxp3 + regulatory T cells impairs mouse melanoma growth. J Exp Med 2012;209:2001-16. [Crossref] [PubMed]
- Chao JL, Savage PA. Unlocking the complexities of tumor-associated regulatory T cells. J Immunol 2018;200:415-21. [Crossref] [PubMed]
- Seminerio I, Descamps G, Dupont S, et al. Infiltration of FoxP3+ regulatory T cells is a strong and independent prognostic factor in head and neck squamous cell carcinoma. Cancers (Basel) 2019;11:227. [Crossref] [PubMed]
- Kayser G, Schulte-Uentrop L, Sienel W, et al. Stromal CD4/CD25 positive T-cells are a strong and independent prognostic factor in non-small cell lung cancer patients, especially with adenocarcinomas. Lung Cancer 2012;76:445-51. [Crossref] [PubMed]
- Kinoshita T, Ishii G, Hiraoka N, et al. Forkhead box P3 regulatory T cells coexisting with cancer associated fibroblasts are correlated with a poor outcome in lung adenocarcinoma. Cancer Sci 2013;104:409-15. [Crossref] [PubMed]
- Sato M, Watanabe S, Tanaka H, et al. Retrospective analysis of antitumor effects and biomarkers for nivolumab in NSCLC patients with EGFR mutations. Nishikawa H, editor. PLoS One 2019;14:e0215292.
- Asai N, Kubo A, Suzuki S, et al. CCR4 Expression in tumor-infiltrating regulatory T cells in patients with squamous cell carcinoma of the lung: a prognostic factor for relapse and survival. Cancer Invest 2019;37:163-73. [Crossref] [PubMed]
- Zhang GQ, Han F, Fang XZ, et al. CD4+, IL17 and Foxp3 expression in different pTNM stages of operable non-small cell lung cancer and effects on disease prognosis. Asian Pac J Cancer Prev 2012;13:3955-60. [Crossref] [PubMed]
- Tao H, Mimura Y, Aoe K, et al. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer 2012;75:95-101. [Crossref] [PubMed]
- Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol 2005;35:1681-91. [Crossref] [PubMed]
- Allan SE, Crome SQ, Crellin NK, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol 2007;19:345-54. [Crossref] [PubMed]
- Tsim S, O’Dowd CA, Milroy R, et al. Staging of non-small cell lung cancer (NSCLC): a review. Respir Med 2010;104:1767-74. [Crossref] [PubMed]
- Schmidt LH, Biesterfeld S, Kümmel A, et al. Tissue microarrays are reliable tools for the clinicopathological characterization of lung cancer tissue. Anticancer Res 2009;29:201-9. [PubMed]
- von Wasielewski R, Mengel M, Wiese B, et al. Tissue array technology for testing interlaboratory and interobserver reproducibility of immunohistochemical estrogen receptor analysis in a large multicenter trial. Am J Clin Pathol 2002;118:675-82. [Crossref] [PubMed]
- Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015;26:259-71. [Crossref] [PubMed]
- Schmidt LH, Kümmel A, Görlich D, et al. PD-1 and PD-L1 expression in NSCLC indicate a favorable prognosis in defined subgroups. PLoS One 2015;10:e0136023. [Crossref] [PubMed]
- Fedchenko N, Reifenrath J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue – a review. Diagn Pathol 2014;9:221. [Crossref] [PubMed]
- Luo Y, Yu T, Zhang Q, et al. Upregulated N-cadherin expression is associated with poor prognosis in epithelial-derived solid tumours: a meta-analysis. Eur J Clin Invest 2018;48:e12903. [Crossref] [PubMed]
- Tsoukalas N, Aravantinou-Fatorou E, Tolia M, et al. Epithelial-mesenchymal transition in non small-cell lung cancer. Anticancer Res 2017;37:1773-8. [Crossref] [PubMed]
- Imai D, Yoshizumi T, Okano S, et al. IFN-γ promotes epithelial-mesenchymal transition and the expression of PD-L1 in pancreatic cancer. J Surg Res 2019;240:115-23. [Crossref] [PubMed]
- Vahl JM, Friedrich J, Mittler S, et al. Interleukin-10-regulated tumour tolerance in non-small cell lung cancer. Br J Cancer 2017;117:1644-55. [Crossref] [PubMed]
- Silva MA, Ryall KA, Wilm C, et al. PD-L1 immunostaining scoring for non-small cell lung cancer based on immunosurveillance parameters. PLoS One 2018;13:e0196464. [Crossref] [PubMed]
- Sterlacci W, Fiegl M, Juskevicius D, et al. Cluster analysis according to immunohistochemistry is a robust tool for non-small cell lung cancer and reveals a distinct, immune signature-defined subgroup. Appl Immunohistochem Mol Morphol 2020;28:274-83. [Crossref] [PubMed]
- Edlund K, Madjar K, Mattsson JSM, et al. Prognostic impact of tumor cell programmed death ligand 1 expression and immune cell infiltration in NSCLC. J Thorac Oncol 2019;14:628-40. [Crossref] [PubMed]
- Lin Z, Gu J, Cui X, et al. Deciphering microenvironment of NSCLC based on CD8+ TIL density and PD-1/PD-L1 expression. J Cancer 2019;10:211-22. [Crossref] [PubMed]
- Kinoshita T, Kudo-Saito C, Muramatsu R, et al. Determination of poor prognostic immune features of tumour microenvironment in non-smoking patients with lung adenocarcinoma. Eur J Cancer 2017;86:15-27. [Crossref] [PubMed]
- Peng J, Yu Z, Xue L, et al. The effect of foxp3-overexpressing Treg cells on non-small cell lung cancer cells. Mol Med Rep 2018;17:5860-8. [PubMed]
- Shang B, Liu Y, Jiang S, et al. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep 2015;5:15179. [Crossref] [PubMed]
- Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother 2011;60:909-18. [Crossref] [PubMed]


