
Spread through air spaces affects survival and recurrence of patients with clinical stage IA non-small cell lung cancer after wedge resection
Introduction
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer-related death worldwide (1). The significance of low-dose CT screening for lung cancer detection has been recognized (2), and small-sized lung cancers are being found more frequently. Sublobar resection is considered an alternative to lobectomy for early-stage non-small cell lung cancer (NSCLC) or compromise NSCLC cases. Since randomized control studies comparing lobectomy with sublobar resection for small-sized lung cancers are ongoing, and the results have not yet been shown (3,4), lobectomy remains the standard surgical treatment, based on a previous randomized control study (5). On the other hand, sublobar resection is associated with lower morbidity rates, a shorter hospital stay, and better preservation of pulmonary function compared with lobectomy (6).
Among the sublobar resection methods, WR is non-anatomical resection often indicated for peripheral small-sized lung cancers, whereas segmentectomy is anatomical resection, preferred for centrally located lung cancers. WR is associated with a shorter operation time, less blood loss, and fewer postoperative complications (7). On the other hand, WR could not dissect more lymph nodes than segmentectomy (7). Overall survival (OS) was investigated according to the surgical procedure (lobectomy, segmentectomy, and WR), and WR was not found to be superior to segmentectomy or lobectomy in terms of an OS benefit (8). A shortcoming of WR is that a sufficient margin for preventing local recurrence cannot be obtained.
Spread through air spaces (STAS) is recognized as a type of lung cancer invasion (9-17). Despite some criticism (18,19), recent studies on NSCLC have shown that STAS is a significant risk factor for recurrence and a prognostic factor for poor OS, especially after sublobar resection (10,20-23). We hypothesized that because WR cannot obtain sufficient margins, STAS predicts a much worse prognosis for patients undergoing WR than for those undergoing segmentectomy. The purpose of this study was to clarify whether STAS is a risk factor for survival and recurrence in patients with NSCLC after WR.
Methods
This was a retrospective study using our institution’s prospectively maintained database of lung cancer patients. The personal data of the patients were anonymized. Our institutional ethics committee approved this study and waived the need for informed consent, since the patient data remained anonymous (institutional review board no. 53).
Study design
Our prospectively collected database was established in May 2004 for patients undergoing surgery for lung cancer (13,21). Our database included the following data: (I) patient demographics (age, sex, smoking status, medical history, body mass index, tumor markers, comorbidities, and pulmonary function tests); (II) radiological findings (maximum tumor size, solid tumor component size, and maximum standardized uptake value on positron emission tomography (PET)/computed tomography (CT); (III) preoperative diagnosis; (IV) clinical and pathological NSCLC stage; (V) surgical procedures; (VI) pathological findings; (VII) complications; and (VIII) outcomes (site of recurrence, death, and follow-up). We used a subset of these patients in our previous paper (13,21). The database was reviewed weekly by the authors and medical assistants.
Patients
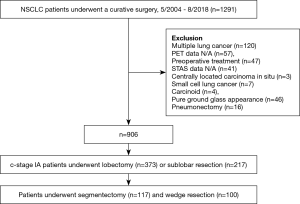
Between May 2004 and August 2018, 1,291 patients underwent complete resection for lung cancer. Of these, 341patients were excluded based on the following exclusion criteria: presence of multiple lung cancers, PET/CT data not available, underwent neoadjuvant therapy, STAS data not available, centrally located squamous cell carcinoma in situ, small cell lung cancer, carcinoids, pure ground grass opacity (GGO) on chest CT, and underwent pneumonectomy. Of the remaining patients, those who underwent WR and segmentectomy were selected. Figure 1 shows the patient enrollment process for this study. A total of 590 patients with clinical stage IA NSCLC who underwent sublobar resection were eligible. Clinical staging was based on the eighth edition of the TNM staging system (24). Cases staged by the seventh edition of the TNM staging system were retrospectively staged using the eighth edition.
Surgery
Lobectomy is the standard procedure for NSCLC. If the patient had a poor performance status, impaired respiratory function, and/or severe comorbidities, segmentectomy or WR was indicated. Intentional WR or segmentectomy was performed for patients with small (≤2 cm) GGO-dominant tumors. WR was performed for peripheral tumors, and segmentectomy was performed for tumors located in the hilum or when sufficient surgical margins could not be obtained by WR. We usually performed segmentectomy via ~10-cm thoracotomy and WR via ~5-cm mini-thoracotomy. Finger palpation was mandatory to obtain a sufficient surgical margin width and to assess the tumor location, and we aimed for a margin of at least 1 cm. In WR, the lung parenchyma was resected using a surgical stapler without identification of the bronchovascular structures. In segmentectomy, after identification and isolation of the tumor and separation of the bronchovascular structures, the intersegmental plane was resected by electrocautery or a surgical stapler. After WR, intraoperative lavage cytology was performed to evaluate the surgical margins. Lymph node dissection or sampling was routinely performed with segmentectomy, but not with WR. If intraoperative frozen section diagnosis revealed that the hilar lymph node was positive during segmentectomy, this procedure was converted to lobectomy.
Radiological evaluation
To measure the maximum tumor size and solid tumor size, we performed thin-section CT using the Aquilion ONE system (Toshiba Medical Systems, Tochigi, Japan) or Lightspeed VCT (General Electric, Milwaukee, WI, USA). The scanning parameters included a tube voltage of 120 kV, tube current of 100–400 mA, and a pitch of 0.028 for Aquilion ONE and 1.3 for Lightspeed VCT. Thin-section images were reconstructed using a 0.5-mm thickness and 0.5-mm reconstruction interval. The maximum tumor and solid component sizes on CT were determined by discussion of the results among physicians, thoracic surgeons, and radiologists. We defined the solid parts of the tumor as those areas that completely obscured the lung parenchyma and the non-solid parts as those areas with increased lung density but through which normal parenchymal structures, such as the bronchus and vessels, could be recognized.
Pathological examination
The surgically resected specimens were fixed in 10% formalin, sectioned into slices of 5–10 mm thickness, and stained with hematoxylin and eosin. For the histopathological examination, we used the 2015 World Health Organization classification (25). We routinely examined the presence of pleural, lymphatic, and vascular invasion and STAS. STAS was defined as detached tumor cells within the air spaces of the lung parenchyma beyond the edge of the main tumor (11,13,21,25). We regarded single-cell clusters near the tumor as artificially detached cell clusters, and not as STAS. STAS was evaluated microscopically by two of the authors (S.S. and N. Y.) in the largest-diameter tumor section.
Postoperative follow-up
The postoperative follow-up schedule consisted of a visit at 1 or 2 weeks after surgery and every month thereafter for up to 3 months. Follow-up was performed every 6 months for 5 years. During the 5-year follow-up, the patients underwent chest CT every 6 months. PET/CT or brain CT imaging was performed in the patients with suspected recurrence with or without symptoms. The date and diagnosis of recurrence were determined according to the consensus of the multidisciplinary team. Local recurrence was defined as tumor recurrence in a contiguously anatomical site, including the ipsilateral hemithorax and mediastinum, after surgical resection. Distant recurrence was defined as tumor recurrence in the contralateral lung or outside the hemithorax and mediastinum after surgical resection. If a biopsy specimen was available, pathological confirmation was performed.
Statistical analysis
Categorical variables are presented as percentages and continuous variables as medians and interquartile ranges. The chi-squared test was used to evaluate the associations between categorical variables and the presence of STAS. Analysis of variance was used to compare continuous variables according to the presence of STAS. The follow-up period was defined as the time from the date of surgery to the date of the last hospital visit or death from any cause. The median follow-up duration, OS, and freedom from recurrence (FR) were estimated using the Kaplan–Meier method. OS was measured from the date of surgery to the date of death from any cause, or censored at the date of the patient’s last hospital visit. FR was measured from the date of surgery to the date of recurrence, and other causes of death were censored. Survival differences were assessed using the log-rank test. Univariate and multivariate Cox proportional hazards regression analyses were performed to identify prognostic factors. Factors that were significant (P<0.05) in the univariate analyses were evaluated in the multivariate analysis. To identify preoperative factors predictive of STAS in the multivariate analysis, receiver operating characteristic analysis was performed to identify the appropriate cut-off values for each factor. Data were analyzed using JMP software, version 11.0.0 (SAS Institute Inc., Cary, NC, USA). A P<0.05 was considered to indicate statistical significance.
Results
Patient demographics
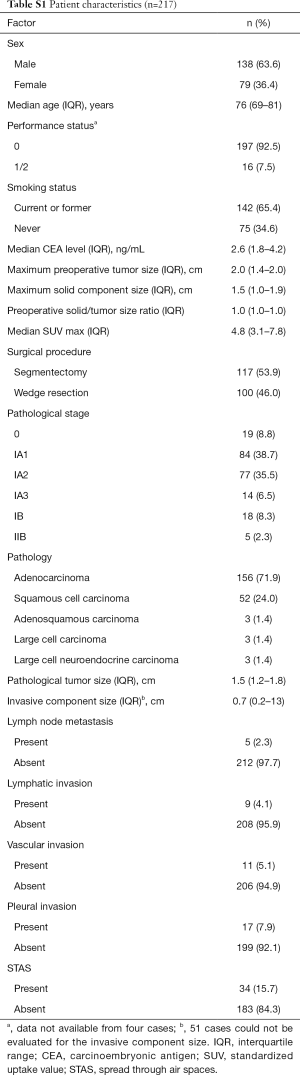
The demographic characteristics of all patients who underwent sublobar resection are shown in Table S1. Of the 217 patients, 100 (46.1%) underwent WR and 117 (53.9%) segmentectomy. Among the WR cases, lymph node evaluation was not performed in 93, hilar lymph node dissection in 3, and lymph node sampling in 4. Among the segmentectomy cases, mediastinal lymph node dissection was performed in 34, hilar lymph node dissection in 82, and lymph node sampling in 1. Of the 217 total patients, 4 (1.8%) had hilar lymph node metastasis, and none had mediastinal lymph node metastasis. STAS was detected in 34 of the 217 (15.7%) cases.
Full table
Surgical outcomes and recurrence
No mortalities were observed after surgery. Of the 217 patients, 36 (16.6%) developed recurrence: 26 (26.0%) after WR and 10 (8.5%) after segmentectomy. The rate of recurrence was significantly higher in the WR than segmentectomy group (P<0.001). Recurrence was local in 21 (21.0%) and 5 (4.3%) patients of the WR and segmentectomy groups, respectively, and this difference was significant (P<0.001). In the distant metastasis, there was no difference in the rate of recurrence between the two procedures (Table S2). According to the presence of STAS, local recurrence occurred in 9 (26.5%) patients with STAS and 17 (9.3%) without STAS, with a significant difference in the rate of local recurrence according to the presence of STAS (P=0.005). In the distant metastasis, there was no difference in the rate of recurrence according to the presence of STAS (P=0.402) (Table S3).

Full table
Full table
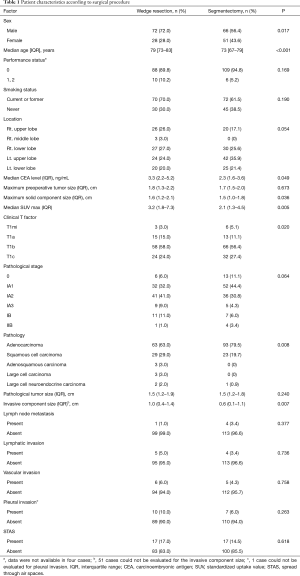
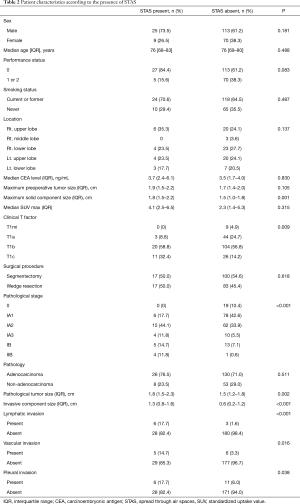
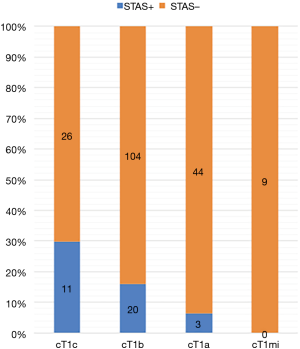
Table 1 shows the patient demographics of the patients according to the surgical procedure (WR versus segmentectomy). The patients who underwent segmentectomy comprised more females, were younger in age, and had a lower serum carcinoembryonic antigen level, lower standardized uptake value, and smaller solid tumor component size compared with the patients who received WR. The patients who underwent segmentectomy had earlier-stage disease, higher rate of adenocarcinoma, and smaller invasive component size. The frequency of STAS was the same between the groups. The clinicopathological characteristics of the patients according to the presence of STAS are provided in Table 2. There was no difference in the frequency of WR or segmentectomy between patients with STAS and those without STAS. Patients with STAS had a larger solid component size on chest CT and a larger pathological tumor size. Pathological invasive factors were more common in patients with STAS than in those without STAS. Whereas STAS was not present in the clinical T1mi (solid tumor size ≤ 5 mm) cases, it was present in 11 of 37 (29.7%) clinical T1c (solid tumor size 2.1–3 cm) cases.
Full table
Full table
Prognostic factors for OS and FR
The median follow-up of the 217 patients was 54 months. Among all study patients, the 5-year OS rate was 70.4% and the 5-year FR rate 76.8%. The 5-year OS rates in the segmentectomy and WR groups were 77.7% and 60.4%, respectively, with a significantly better OS rate in the segmentectomy than WR group (P=0.002). The 5-year FR rates in the segmentectomy and WR groups were 85.8% and 60.8%, respectively, with a significantly better FR rate in the segmentectomy than WR group (P<0.001) (Figure S1).
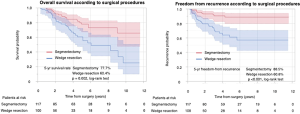
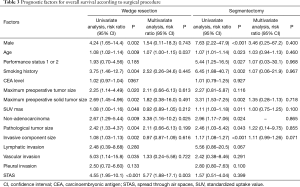
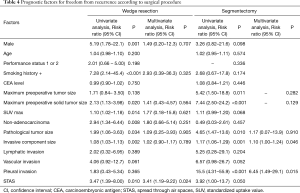
We assessed the relationships between OS and clinicopathological factors according to surgical procedure. Tables 3,4 show the results of univariate and multivariate analyses of prognostic factors for OS and FR, respectively, in the WR and segmentectomy groups. Multivariate analysis revealed that age, histology, and STAS were significant factors predicting OS in the WR group (Table 3). However, in the segmentectomy group, none of the factors evaluated in the multivariate analysis were significant (Table 3). Regarding FR, only STAS was identified as a significant predictor of recurrence in the WR group, whereas the solid tumor component size on CT and pleural invasion were significant factors predicting recurrence in the segmentectomy group (Table 4). STAS was marginally significant as a predictive factor in the univariate analysis, but was not significant in the multivariate analysis (P=0.145), for FR in the segmentectomy group. We categorized the patients according to both the surgical procedure (WR versus segmentectomy) and the presence versus absence of STAS. Figure 2 shows Kaplan-Meier curves for OS (Figure 2A) and FR (Figure 2B) rates for NSCLC patients after surgery according to the presence or absence of STAS and according to the surgical procedure, and the patients with STAS who underwent WR had the worst OS (Figure 2A) and FR (Figure 2B) rates in four groups.
Full table
Full table

Prediction of STAS
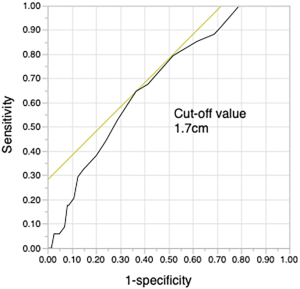
As our results showed that the maximum size of the solid tumor component on CT was significantly related to STAS, a cut-off size of 1.7 cm was determined by receiver operating characteristic analysis (Figure S2). STAS was more likely to be present when the solid tumor size was ≥1.7 cm. Since the solid tumor size on CT is associated with the T stage (TNM classification, eighth edition) (24), the presence of STAS was classified by T stage (Figure 3).
Discussion
The CALGB/Alliance 140503 and JCOG0802/WJOG4607L trials aimed to evaluate the non-inferiority of sublobar resection to lobectomy in terms of survival in patients with NSCLC (3,4). Among the sublobar resection methods, WR and segmentectomy are non-anatomical and anatomical resections, respectively. These procedures are not equivalent technically or oncologically. Altorki et al. showed that the surgical outcomes of segmentectomy and WR were comparable in patients with cT1N0 NSCLC (7). In a retrospective analysis of 6,905 stage I NSCLC patients, OS after lobectomy, segmentectomy, and WR was assessed using propensity score matching. The 5-year OS rates were 58.1%, 78.3%, and 79.1% after WR, segmentectomy, and lobectomy, respectively, with a significantly worse rate after WR than after segmentectomy and lobectomy. However, after propensity score matching, the hazard ratio of survival for segmentectomy compared with WR was 0.67 and was not statistically significant (P=0.101) (8). Even though our study showed that segmentectomy was superior to WR in terms of OS and FR, WR might be comparable with segmentectomy if the appropriate patients are selected for each treatment.
STAS is recognized as a type of lung cancer invasion and is considered a significant risk factor for recurrence after sublobar resection of lung cancer (10,20-23). Eguchi et al. investigated 1,497 patients who underwent lobectomy or sublobar resection for pathological T1N0M0 lung adenocarcinoma using propensity score matching. They found that the patients with STAS had higher risks of recurrence and cancer-specific death after sublobar resection (23). The major advantages of segmentectomy over WR are the ability to obtain greater surgical margins and to evaluate more lymph nodes. We hypothesized that because WR cannot obtain a sufficient margin width, STAS has a much worse prognostic impact on patients undergoing WR compared with segmentectomy. We showed that STAS was a significant prognostic factor for patients with clinical stage IA NSCLC who underwent WR, but not for those who underwent segmentectomy. A possible explanation for this result is that the wider surgical margin with segmentectomy prevents the spread of tumor cells, i.e., STAS. In NSCLC with STAS, the tumor cell clusters floating around the primary tumor have the potential to attach and grow in the surgical stump or other parts of the lung. However, it is not understood how these floating tumor cell clusters survive without a blood supply. Tanaka et al. demonstrated intrabronchial implantation of human adenocarcinoma cells in a mouse model of severe combined immunodeficiency disease (26). Although the same mechanism has not been observed in the human lung, those findings suggest that STAS potentially promotes tumor implantation or metastasis.
The greatest concern after sublobar resection is local recurrence, which occurs in approximately 20% of cases (11,27), which was similar to our results. The surgical margin width is thought to be related to local recurrence. In the present study, the surgical margin width could be evaluated in 65 patients, among whom the mean margin was 0.9 cm in those with local recurrence and 1.1 cm in those without local recurrence (P=0.106), with no significant difference according to the presence of recurrence. Goldstein et al. investigated patients who underwent WR followed by lobectomy and revealed that those with residual adenocarcinoma after WR had a shorter surgical margin distance compared with those cases without residual tumor (0.7 vs. 2.4 mm) (28). Altorki et al. demonstrated an optimal cut-off margin size of ≥1.0 cm (7). Schuchert et al. suggested that a margin/tumor ratio < 1 is associated with a higher rate of recurrence (29). However, the results of ACOSOG Z4032 compared sublobar resection with intraoperative brachytherapy with sublobar resection alone showed that a surgical margin distance <1.0 cm, margin/tumor ratio <1.0, positivity on staple cytology, WR, and a clinical nodule size >2.0 cm were not associated with local recurrence (30). The lack of a standard method to measure the surgical margin during sublobar resection could explain the controversial results regarding the surgical margin. Moreover, because of air in the lung parenchyma, measuring the surgical margin width may be difficult.
Almost all pulmonary segments can be accessed using segmentectomy, but WR has limited access depending on the tumor location. For example, deep fissures and the base of the tumor are located in difficult to resect regions for WR (31). Thus, insufficient surgical margins would lead to a high risk of developing local recurrence. Masai et al. demonstrated that a surgical margin <1.0 cm and STAS are significantly related to local recurrence (20).
Prediction of STAS is essential for preventing recurrence. Some studies have suggested that the size of the solid tumor component is related to the presence of STAS (13,32,33). Toyokawa et al. showed that, on the pulmonary nodule, the presence of notch and the absence of GGO on chest CT were also significantly associated with the presence of STAS (32). Kim et al. investigated adenocarcinoma of greater than T1 stage and revealed that the presence of STAS was significantly related to central low attenuation, ill-defined opacity, and presence of an air bronchogram on chest CT (33). Preoperative radiological findings such as notch or central attenuation were also predictive of STAS, but there are concerns with the consistency of these features and discrepancies in their evaluation among observers. Simpler indicators predicting STAS are needed. In the current study, we clearly demonstrated that a cut-off solid tumor size of 1.7 cm was predictive of STAS. Furthermore, the patients with stage cT1mi on preoperative CT did not have STAS. Since the solid tumor size on CT is an important element of the eighth edition of the TNM classification (24,34), WR may be acceptable for resection of cT1mi NSCLC based on these results. In the case of clinical stage IA NSCLC with a solid tumor size on CT ≥1.7 cm, segmentectomy or lobectomy is a reasonable approach to obtain a wider surgical margin.
Several limitations of this study must be acknowledged. First, although we emphasized the significance of the surgical margin, we were unable to show sufficient information on the surgical margin width. Second, because the background characteristics differed between the patients who underwent WR and those who underwent segmentectomy, selection bias may have been introduced, and the patients who underwent WR were frailer and had more invasive NSCLC. To confirm our results, further analysis of segmentectomy cases is needed. Third, this was a retrospective single-center study. However, we used a prospectively maintained database and the standard definition of STAS. A multi-institutional prospective study could provide clarification of how STAS affects the surgical outcome.
In conclusion, we showed that STAS was a prognostic factor for patients undergoing WR. Patients with clinical stage IA NSCLC with a solid tumor size on CT of ≥1.7 cm tended to have STAS. Patient selection is important, and it is crucial to keep in mind that WR should be performed only if clearly indicated to prevent recurrence via STAS.
Acknowledgments
These findings were presented at the AATS International Thoracic Surgical Oncology Summit, New York, September 27–28, 2019.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.04.47). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our institutional ethics committee approved this study and waived the need for informed consent, since the patient data remained anonymous (institutional review board no. 53).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915-24. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- De Zoysa MK, Hamed D, Routledge T, et al. Is limited pulmonary resection equivalent to lobectomy for surgical management of stage I non-small cell lung cancer? Interact Cardiovasc Thorac Surg 2012;14:816-20. [Crossref] [PubMed]
- Altorki NK, Kamel MK, Narula N, et al. Anatomical segmentectomy and wedge resections are associated with comparable outcomes for patients with small cT1N0 non-small cell lung cancer. J Thorac Oncol 2016;11:1984-92. [Crossref] [PubMed]
- Dziedzic R, Zurek W, Marjanski T, et al. Stage I non-small-cell lung cancer: long-term results of lobectomy versus sublobar resection from the Polish National Lung Cancer Registry. Eur J Cardiothorac Surg 2017;52:363-9. [Crossref] [PubMed]
- Warth A, Muley T, Kossakowski CA, et al. Prognostic impact of intra-alveolar tumor spread in pulmonary adenocarcinomas. Am J Surg Pathol 2015;39:793-801. [Crossref] [PubMed]
- Onozato ML, Kovach AE, Yeap BY, et al. Tumor islands in resected early stage lung adenocarcinomas are associated with unique clinicopathological and molecular characteristics and worse prognosis. Am J Surg Pathol 2013;37:287-94. [Crossref] [PubMed]
- Kadota K, Nitadori J, Sima CS, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Morimoto J, Nakajima T, Suzuki H, et al. Impact of free tumor clusters on prognosis after resection of pulmonary adenocarcinoma. J Thorac Cardiovasc Surg 2016;152:64-72.e1. [Crossref] [PubMed]
- Shiono S, Yanagawa N. Spread through air spaces is a predictive factor of recurrence and a prognostic factor in stage I adenocarcinoma. Interact Cardiovasc Thorac Surg 2016;23:567-72. [Crossref] [PubMed]
- Lu S, Tan KS, Kadota K, et al. Spread through air spaces (STAS) is an independent predictor of recurrence and lung cancer-specific death in squamous cell carcinoma. J Thorac Oncol 2017;12:223-34. [Crossref] [PubMed]
- Uruga H, Fujii T, Fujimori S, et al. Semiquantitative assessment of tumor spread through air spaces (STAS) in early-stage lung adenocarcinomas. J Thorac Oncol 2017;12:1046-51. [Crossref] [PubMed]
- Dai C, Xie H, Su H, et al. Tumor spread thorough air spaces affects the recurrence and overall survival in patients with lung adenocarcinoma >2 to 3 cm. J Thorac Oncol 2017;12:1052-60. [Crossref] [PubMed]
- Kadota K, Kushida Y, Katuski N, et al. Tumor spread through air spaces is an independent predictor of recurrence-free survival in patients with resected lung squamous cell carcinoma. Am J Surg Pathol 2017;41:1077-86. [Crossref] [PubMed]
- Blaauwgeers H, Flieder D, Warth A, et al. A prospective study of loose tissue fragments in non-small cell lung cancer resection specimens. An alternative view to “spread through air spaces”. Am J Surg Pathol 2017;41:1226-30. [Crossref] [PubMed]
- Blaauwgeers H, Russell PA, Jones KD, et al. Pulmonary loose tumor tissue fragments and spread through air spaces (STAS): Invasive pattern or artifact? A critical review. Lung Cancer 2018;123:107-11. [Crossref] [PubMed]
- Masai K, Sakurai H, Sukeda A, et al. Prognostic impact of margin distance and tumor spread through air spaces in limited resection for primary lung cancer. J Thorac Oncol 2017;12:1788-97. [Crossref] [PubMed]
- Shiono S, Endo M, Suzuki K, et al. Spread through air spaces is a prognostic factor in sublobar resection of non-small cell lung cancer. Ann Thorac Surg 2018;106:354-60. [Crossref] [PubMed]
- Bains S, Eguchi T, Warth A, et al. Procedure-specific risk prediction for recurrence in patients undergoing lobectomy or sublobar resection for small (≤2 cm) lung adenocarcinoma: an international cohort analysis. J Thorac Oncol 2019;14:72-86. [Crossref] [PubMed]
- Eguchi T, Kameda K, Lu S, et al. Lobectomy Is associated with better outcomes than sublobar resection in spread through air spaces (STAS)-positive T1 lung adenocarcinoma: a propensity score-matched analysis. J Thorac Oncol 2019;14:87-98. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer, 2015.
- Tanaka R, Tachibana K, Suda K, et al. A severe combined immunodeficiency disease mouse model of human adenocarcinoma with lepidic-predominant growth. Pathol Res Pract 2018;214:2000-3. [Crossref] [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Impact of brachytherapy on local recurrence rates after sublobar resection: results from ACOSOGZ4032 (Aliance), a phase III randomized trial for high-risk operable non-small cell lung cancer. J Clin Oncol 2014;32:2456-62. [Crossref] [PubMed]
- Goldstein NS, Ferkowicz M, Kestin L, et al. Wedge resection margin distances and residual adenocarcinoma in lobectomy specimens. Am J Clin Pathol 2003;120:720-24. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:926-32. [Crossref] [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Thirty- and ninety-day outcomes after sublobar resection with and without brachytherapy for non-small cell lung cancer: results from a multicenter phase III study. J Thorac Cardiovasc Surg 2011;142:1143-51. [Crossref] [PubMed]
- Lewis RJ, Caccavale RJ, Sisler GE, et al. Video-assisted thoracic surgical resection of malignant lung tumors. J Thorac Cardiovasc Surg. 1992;104:1679-85. [Crossref] [PubMed]
- Toyokawa G, Yamada Y, Tagawa T, et al. Computed tomography features of resected lung adenocarcinomas with spread through air spaces. J Thorac Cardiovasc Surg 2018;156:1670-6.e4. [Crossref] [PubMed]
- Kim SK, Kim TJ, Chung MJ, et al. Lung adenocarcinoma: CT features associated with spread through air spaces. Radiology 2018;289:831-40. [Crossref] [PubMed]
- Kameda K, Eguchi T, Lu S, et al. Implications of the eight edition of the TNM proposal: invasive versus total tumor size for the T descriptor in pathologic stage I-IIA lung adenocarcinoma. J Thorac Oncol 2018;13:1919-29. [Crossref] [PubMed]


