
This article has an erratum available at: http://dx.doi.org/10.21037/jtd-2021-40 the article has been update on 2021-10-26 at here.
Internal fixation of the proximal tracheal self-expandable metallic stent (SEMS): migration prevention in high risk patients
Introduction
Bronchoscopic stent insertion represents a chosen treatment for patients presented with malignant central airways stenosis with a palliative purpose to re-establish airway patency and provide an immediate and significant relief of the patient’s symptoms (1).
Self-expanding metallic airways stents (SEMS) represents a standard method of airways stenting especially when employed for the management of malignant central airway obstruction (2).
Despite the obvious stenting advantages, it may be complicated with stent migration especially in high tracheal stenosis. The incidence of stent migration is estimated as 5–17% and it is more common in tracheal than bronchial lesions (3).
In this report we describe a case series of internal fixation of SEMS which were employed in three patients with high tracheal stenosis due to different causes with the main aim to prevent the frequently occurring stent migration.
Operative techniques
Technique
After fulfillment of the clinical and radiological indications of the tracheal stent insertion, the patients were recruited for rigid bronchoscopic examinations under general anesthesia. The intervention was done through tracheoscopy (Karl Storz, 33 cm long and 12 mm). The flexible bronchoscope was introduced through the rigid bronchoscope. Firstly, careful bronchoscopic evaluation was performed to estimate the length and the diameter of the stenosed portion of the trachea, therefore upon that evaluation the size of the stent (including the length and diameter) could be determined.
Once the suitable stent was chosen, it was inserted under fluoroscopic control as usual. Both the distal and proximal portions of the stenosis were marked under the fluoroscopy and flexible bronchoscope was removed and the stent delivery device was introduced through the rigid bronchoscope. Under fluoroscopic guidance, the stent was released to re-expand the target stenosed portion and followed up by bronchoscopic confirmation of the exact position of the stent. A rigid optic forceps (10350 L, Karl Storz, Germany) was used as a needle holder to grasp the needle (Mersilene FSL 0 cutting, braided, Polyester, length: 75 cm) through the rigid bronchoscope till it passes though both posterior tracheal wall and together through the stent.
Both two external free ends of the suture were tied together carefully, then the knot was pulled throughout the rigid bronchoscope using the rigid forceps till it was fixed firmly at the proximal end of the stent. This step was repeated to perform 2 or 3 knots to secure the fixation of the stent in its position (Figure 1).
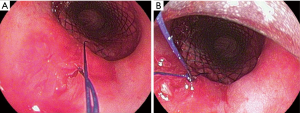
The internal fixation, which we described in this report is a simple intratracheal suture to fix the proximal end of the stent to the posterior wall of the trachea The internal fixation was utilized in fewer reports and up to our knowledge all of them were silicon stents (4,5). It is technically difficult and should be performed by a highly-skilled bronchoscopist who can manipulate the different tools through the rigid bronchoscopy to be able to take the suture through the tracheal mucosa together through the stent.
The duration of the stent fixation was not recorded in our three cases. It was compared in both external and internal approaches to fix a silicon stents in the same patient (4). It was shorter in the external fixation than that of the internal one. In our patients, it takes a lot of time to perform the suture and to finish the fixation after several trials, this reflects the need for developing new bronchoscopic tools that could be used through the rigid bronchoscopy. Based on the thinner wall of the SEMS as well as the relative lower force needed to penetrate the fenestrated metal stents with the suture needle in comparison to the silicon one, we are expecting a shorter procedural duration after gaining more experience and familiarity with the technique. In addition to developing refined tools to hold the needle through the rigid bronchoscopy down to fix the stents.
The internal fixation overcomes the drawbacks of the external fixation including avoidance of puncture of neck and trachea either in one or two points (6), skin maceration with recurrent infections or abscess formation (7), swallowing discomfort (7) and difficulty to remove the suture when they are deeply hidden in the subcutaneous tissue (1). Theoretically, Silicon stent tearing or cracking due to dragging of the sutures or forcible insertion of the needle through the stent can occur. However, till now was not reported (1).
The possibility of easy removal of both the suture and stent, remain an advantage in our approach. It was done in two patients, after 6 weeks and 7 months without complications.
In our report we describe our techniques which was performed as detailed in the following.
Case 1
An 88 years old female was presented with a high-grade tracheal stenosis due to compression by a large mediastinal mass which was later on diagnosed as a non-Hodgkin lymphoma. For symptomatic relief from the presence of stridor before chemotherapy induction, a tracheal stent insertion was decided. A subglottic covered tracheal stent (Micro-Tec ST05-103.18.050) was inserted and concurrently internally fixed using Mersilene suture to prevent its migration (Figure 2).
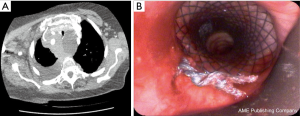
Six weeks after induction of the chemotherapy (R-mini- CHOP protocol), the stent could be successfully extracted after removal of the suture with a sufficient tracheal lumen behind.
Case 2
A female patient, 66 years old presented with symptomatic tracheal stenosis due compression by esophageal adenocarcinoma (Figure 3A). A covered stent (Micro-Tec ST05-103.18.050) was inserted without complications, however during the balloon dilatation, the stent was dislocated distally and was pulled up twice after the balloon dilation. Due to stent instability, internal suturing of the stent was performed using Mersilene suture. A follow up bronchoscope showed that the stent remained in situ (Figure 3B).
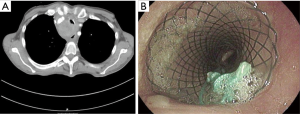
Case 3
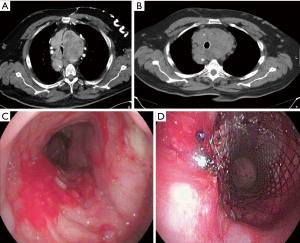
A male patient, 74 years old was referred to our center for stent insertion to achieve a symptomatic relief of tracheal stenosis due to presence of large retrosternal goiter, which was inoperable due to recent history of unstable angina, which was treated with coronary artery stenting and dual antiplatelets medications. Bronchoscopically, the tracheal lumen was stenosed due to extraluminal compression with a lumen diameter of approximately 5 mm. The covered stent (Micro-Tec ST05-103.20.050) was successfully inserted. However, the follow up bronchoscopy that was done two days later, showed that the stent migrated distally. Therefore, another bronchoscope was performed and the stent was fixed using Prolene suture. After 7 months, the planned thyroidectomy was done through sternotomy. One week after the operation, a rigid bronchoscopy was performed. The suture was cut and the stent was successfully removed as well as the granulation tissue around its distal end without any complications. Moreover, no tracheal stenosis was observed and the dynamic collapsibility was significantly decreased (Figure 4).
Comments
The management of malignant tracheal stenosis is a highly challenging which warrant a multidisciplinary approach in every individual case (7). Bronchoscopic stent insertion represents a palliative therapeutic option in symptomatic patients with proximal tracheal stenosis (7), in whom the radical surgical resection is not possible or contraindicated due to medical unfitness or unrespectable lesion depending upon the location and the length of the stenosis (5,6). Tracheal stent provides not only an immediate significant symptomatic and functional improvement but also a preservation of the phonation function and improvement of the quality of life, as well as increases the survival rate of those patients (1,8-11).
SEMS have the following advantages over the silicon stents: firstly, they can be successfully inserted using a flexible bronchoscopy (12). Secondly, low complication rate; including stent migration, low incidence of mucus plug formation or tumor ingrowth (13,14). Thirdly, SEMS have morphologically a thinner wall which provide a larger cross sectional diameter and therefore fits the airways better, making them preferable choice in complex stenosis with irregular airways. Additionally, their adaptation in the airways is improved by their intrinsic radial force which keeps them in position by embedding their ends into the bronchial mucosa (2,15). The disadvantages of the SEMS include: removal difficulties, high price (15), and high rates of granulation tissues formation leading to stent’s obstruction (16). Finally, stent migration was also reported (16).
The incidence of migration was estimated to be 5–17%, with more migration tendency in tracheal- than in bronchial stents (15). In other reports, the 30 days migration rate was in the UltraflexTM 4.7–7.6% and in Wallsten stents 12% (9). Many factors contribute to and increase the risk of migration, especially the nature and site of the lesions. Severe funnel-shaped lesions enhance that risk (2,16). High tracheal stenosis is associated with a high risk of migration especially in lesions within 2 cm distal to the vocal cords (1). The distal migration of the stents will lead to symptomatic recurrence of the original stricture and the proximal migration will induce dysphonia, coughing and series respiratory distress (7). The rationale of the stent fixation is to overcome or to correct the migration especially in high risk lesions, leading to maintaining of the airway patency and reversing of the symptomatic stenosis (1,4). Additionally, the local mucosal irritation induced by the minimal movement of the stent will be reduced after the stent fixation (4). It should be considered in selected patients, whom are inoperable and experienced recurrent stent migrations (7).
In our report, three different situations were described, which could illustrate to some extent the most possible indications of the stent fixation. In the first patient, the decision of the stent fixation was based upon the nature and location of the tracheal stenosis, which reflects a high risk of stent migration. The fixation was prophylactically performed. In the second patient, the decision of stent fixation was made after observation of distal migration during balloon dilation immediately after insertion. Both insertion and fixation were done in the same session. In other words, in both patients, the fixation was done prophylactically to avoid the stent migration and to prevent its occurrence. In the third patient, the stent was fixed to overcome and correct its distal migration, as a classic indication of stent fixation.
Another observation in our work that worth to be mentioned, was the use of the SEMS to manage excessive dynamic airway collapse. A significant improvement of the tracheal collapsibility during expiration was observed after removal of the stent after 7 months, in the third patient without noticeable residual injury of the trachea even during the stent removal. This technique was previously described including the use of silicon or SEMS (17-19). A trail of the airway stenting was also used to assess the response. Those with symptomatic improvement could be considered as surgical candidates (17,20).
Two points still remain questionable, whether the stent fixation should be considered as a routine intervention in patients with complex lesions and high-risk factor, concurrently at the time of the stent insertion or should it be spared as a therapeutic intervention after stent migration? In our report, the concurrent stent insertion and fixation was associated with satisfying clinical and bronchoscopic results. Also, when indicated, the suture as well as the stent could be easily removed.
Finally, despite the low risk of SEMS migration, it was reported specially in proximal complex tracheal lesions representing a possible major complication. Despite its difficultly, the internal fixation of the stent is a feasible method for both overcoming and preventing the stent migration without obvious post procedural complications or patient discomfort. It should be also concurrently considered in selected patients with complex proximal tracheal lesions with high risk of stent migration.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-642). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lin X, Ye M, Li Y, et al. A novel simple external fixation for securing silicone stent in patients with upper tracheal stenosis. J Thorac Dis 2018;10:E194-8. [Crossref] [PubMed]
- Saad CP, Murthy S, Krizmanich G, et al. Self-Expandable Metallic Airway Stents and Flexible Bronchoscopy: Long-term Outcomes Analysis. Chest 2003;124:1993-9. [Crossref] [PubMed]
- Kim JH, Shin JH, Song HY, et al. Benign Tracheobronchial Strictures: Long-Term Results and Factors Affecting Airway Patency After Temporary Stent Placement. AJR Am J Roentgenol 2007;188:1033-8. [Crossref] [PubMed]
- Hohenforst-Schmidt W, Linsmeier B, Zarogoulidis P, et al. Transtracheal single-point stent fixation in posttracheotomy tracheomalacia under cone-beam computer tomography guidance by transmural suturing with the Berci needle - a perspective on a new tool to avoid stent migration of Dumon stents. Ther Clin Risk Manag 2015;11:837-50. [PubMed]
- Temes RT, Wernly JA, Cooper JD, et al. Internal fixation of high tracheal stents. Ann Thorac Surg 1995;59:1023-4. [Crossref] [PubMed]
- Musani AI, Jensen K, Mitchell JD, et al. Novel use of a percutaneous endoscopic gastrostomy tube fastener for securing silicone tracheal stents in patients with benign proximal airway obstruction. J Bronchology Interv Pulmonol 2012;19:121-5. [Crossref] [PubMed]
- Colt HG, Harrell J, Neuman TR, et al. External fixation of subglottic tracheal stents. Chest 1994;105:1653-7. [Crossref] [PubMed]
- Ranu H, Madden BP. Endobronchial stenting in the management of large airway pathology. Postgrad Med J 2009;85:682-7. [Crossref] [PubMed]
- Marchese R, Poidomani G, Paglino G, et al. Fully covered self-expandable metal stent in tracheobronchial disorders: clinical experience. Respiration 2015;89:49-56. [Crossref] [PubMed]
- Amjadi K, Voduc N, Cruysberghs Y, et al. Impact of interventional bronchoscopy on quality of life in malignant airway obstruction. Respiration 2008;76:421-8. [Crossref] [PubMed]
- Murgu S, Langer S, Colt H. Bronchoscopic intervention obviates the need for continued mechanical ventilation in patients with airway obstruction and respiratory failure from inoperable non-small-cell lung cancer. Respiration 2012;84:55-61. [Crossref] [PubMed]
- Kim YH, Shin JH, Song HY, et al. Tracheal stricture and fistula: Management with a barbed silicone-covered retrievable expandable nitinol stent. AJR Am J Roentgenol 2010;194:W232-7. [Crossref] [PubMed]
- Carré P, Rousseau H, Lombart L, et al. Balloon Dilatation and Self-Expanding Metal Wallstent Insertion: For Management of Bronchostenosis Following Lung Transplantation. Chest 1994;105:343-8. [Crossref] [PubMed]
- Brichon PY, Blanc-Jouvan F, Rousseau H, et al. Endovascular stents for bronchial stenosis after lung transplantation. Transplant Proc 1992;24:2656-9. [PubMed]
- Kim YH, Shin JH, Song HY, et al. Tracheal stricture and fistula: management with a barbed silicone-covered retrievable expandable nitinol stent. AJR Am J Roentgenol 2010;194:W232-7. [Crossref] [PubMed]
- Cho SB, Cha SA, Choi JY, et al. Serious Complications after Self-expandable Metallic Stent Insertion in a Patient with Malignant Lymphoma. Tuberc Respir Dis (Seoul) 2015;78:31-5. [Crossref] [PubMed]
- Ernst A, Majid A, Feller-Kopman D, et al. Airway stabilization with silicone stents for treating adult tracheobronchomalacia: a prospective observational study. Chest 2007;132:609-16. [Crossref] [PubMed]
- Mughal MM, Gildea TR, Murthy S, et al. Short-term deployment of self-expanding metallic stents facilitates healing of bronchial dehiscence. Am J Respir Crit Care Med 2005;172:768-71. [Crossref] [PubMed]
- Majid A, Alape D, Kheir F, et al. Short-Term Use of Uncovered Self-Expanding Metallic Airway Stents for Severe Expiratory Central Airway Collapse. Respiration 2016;92:389-396. [Crossref] [PubMed]
- Ochoa S, Cheng GZ, Folch E, et al. Use of Self-expanding Metallic Airway Stents in Tracheobronchomalacia. J Bronchology Interv Pulmonol 2015;22:e9-11. [Crossref] [PubMed]


