How to reduce bleeding complications during thoracoscopic anatomic pulmonary resection
It is a great pleasure for us to comment on the article “Algorithm-based troubleshooting to manage bleeding during thoracoscopic anatomic pulmonary resection,” which was recently published in the Journal of Thoracic Disease (1). First of all, we would like to congratulate Dr. Igai and his colleagues for their great efforts to the clinical study of video-assisted thoracoscopic surgery (VATS) troubleshooting (2,3). We read their most recent study on our journal with great interest. The algorithm they propose is reasonable for almost all thoracic surgeons and is an acceptable way to troubleshoot bleeding. Particularly noteworthy in their paper was their establishment of an algorithm for bleeding in VATS and comparison of the incidence of intraoperative hemorrhage between the early and most recent observation periods. Importantly, they showed that the incidence did not change and that intraoperative hemorrhage was not due to the surgeon’s experience but to a certain probability.
VATS for lung cancer has already been spread based for its good clinical outcome; less invasiveness, including reduced post-thoracotomy pain (4); shorter hospitalization period; and similar oncological outcomes (5) compared with standard thoracotomy. For example, from the annual report of the Japanese Association for Thoracic Surgery (6), 32,206 (73.0%) of 44,140 patients with primary lung cancer underwent VATS in 2017. The annual national investigation of endoscopic surgery by the Japan Society for Endoscopic Surgery showed that the number of VATS performed for lung disease increased constantly, and among 191 facilities that responded, 137 (71.7%) performed complete VATS; 44 (23.0%) in VATS under direct vision, and 68 (35.6%) in hybrid surgery (7). Moreover, the indications for pulmonary resection have expanded to patients of advanced age, patients with severe comorbidities who could not previously tolerate surgery, and patients with conditions requiring difficult procedures, for examples, severe fused fissures, dense adhesions, lymph node swelling, clinical stage II and more, broncho and/or angioplastic resection, chest wall and diaphragm resection (8).
With this widespread use of VATS, another concern has been the safety of VATS. In fact, catastrophic intraoperative complications have been reported, for example, massive bleeding, anatomical misidentifications such as bronchus and pulmonary vessels, contralateral nerve and bronchial injury. The management of these complications is far different from that during thoracotomy. Above all, significant bleeding from the pulmonary artery (PA) sometimes forces surgeons change the procedure (e.g., have to perform pneumonectomy for bleeding control) or manage a fatal situation. Not so many reports have focused on the occurrence and specific troubleshooting of this kind of unfavorable intraoperative bleeding due to its negative results, and it’s left to the surgeon’s discretion how to deal with it.
However, several recent reports have discussed troubleshooting during VATS, especially for bleeding from the PA (8,9). Surgery continues to evolve into minimally invasive surgery over the years, such as robotic and uniportal surgery. Accordingly, troubleshooting protocols for bleeding in these procedures are beginning to be reported (10,11). An international expert consensus on the management of bleeding during VATS lung surgery has been published (12). This report is expected to be very helpful because it presents many ways to deal with bleeding not only from the PA but also from the superior vena cava, lung parenchyma, and chest wall as well as during lymph node dissection. We have also evaluated unexpected bleeding during VATS (8). In our single-institutional study, 20 (8.3%) of 241 patients performed VATS anatomical pulmonary resections were needed hemostatic procedures either vessel sutures or a sealant. The most injured vessels were the PA, and the main reasons of vessel injuries were related to surgeon’s technical problems of the endoscopic instruments. There were no postoperative complications thought to be due to intraoperative bleeding. The differences in the operation time and blood loss volume between patients with and without vessel injury were statistically significant; however, the perioperative morbidities, durations of chest tube insertion, and duration of postoperative hospitalization were not significantly different.
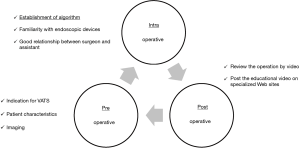
Needless to say, it is important to prevent these serious bleeding events during VATS. Figure 1 shows several preventive measures that we propose. The surgical team must be aware of these preventive measures at each time point (preoperatively, intraoperatively, and postoperatively) in daily clinical practice.
Preoperatively, the patient’s indication for VATS and clinical characteristics, including comorbidities (e.g., chronic obstructive pulmonary disease, pneumoconiosis, tuberculosis, or aspergillosis) and imaging findings (e.g., calcified or infiltrated metastatic lymph nodes, pleural adhesion, or anomalies of the PA, pulmonary vein, and bronchus), are very important factors that affect the complexity of surgery. We have developed and validated a simple aggregate score with which to preoperatively stratify the complexity of VATS lobectomy. This score is based on several clinical factors including male sex, emphysema on chest computed tomography, pleural thickness, and enlarged hilar lymph nodes, and it seemed to be reproducible in another institution. This score might be adapted to identify appropriate patients for VATS lobectomy preoperatively and increase the effectiveness and safety of the training period (13).
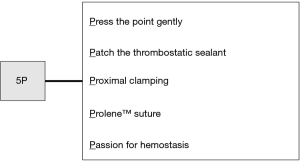
Intraoperatively, each surgical team needs an algorithm for unexpected bleeding. This could contribute to the performance of proper interventions without panic. Endoscopic forceps, energy devices and staplers have become indispensable for VATS and improve surgeons’ performance and safety. Surgeons must carefully perform all maneuvers and ensure sufficient proficiency with all endoscopic instruments. Actually, in our study, the most causes of vessel injuries were related to human errors or careless mistakes, not mechanical troubles (8). The surgeon should always be familiar with the handling of these endoscopic instruments because of their rapid advancement in technology. It is also important to have a good relationship with the surgical assistants so that they can proactively discuss dangerous procedures with the surgeon. We totally agree with the algorithm established by Igai et al.; therefore, we would also like to recommend the following “5P” procedures to control bleeding: “Press the point gently,” “Patch the thrombostatic sealant,” “Proximal clamping,” “Prolene (monofilament non-absorbable) suture,” and “Passion for hemostasis” (Figure 2). Above all, we believe that safety and complete resection must be the most important priorities in every surgery. The need for emergent conversion to thoracotomy does not indicate a failure of the surgery. However, it does mean that backup medical staff should be available in case that the surgical team fails to control the significant bleeding.
Postoperatively, we have been fortunate enough to be able to record and store surgical videos more easily. We should constantly look back at our own surgeries and strive to stabilize and improve our technique. In addition, the video clip of surgery has been attached in recent academic papers (14-17), which is very informative. The vast numbers of surgical procedures that have been shown as videos on specific Internet sites, and we could learn the techniques in wet and dry laboratory seminars (10). We believe that these constant practices will lead to improved and sophisticated VATS technique.
The article by Igai et al. had several limitations. Firstly, this study was conducted in a single center only. The findings should have been validated in an external cohort because the outcomes are also dependent upon the skills of a specific surgeon. Secondly, depending on the extent of the bleeding, we would like to see the authors show how they are educating their trainees to handle this situation. Finally, long-term results are required to determine how this algorithm affects overall survival. We would like the authors to continue to follow up the patients and report their findings again in the future.
In conclusion, although intraoperative bleeding is inherent to the performance of surgery, VATS anatomical pulmonary resection is feasible and safe when the surgical team has the appropriate algorithm for significant bleeding. Surgeons should recognize that conversion should not be regarded as the failure of the minimally invasive surgery but instead another way to keep the safety. Even as minimally invasive surgery continues to advance, this unexpected complication will continue to occur at a certain rate.
Acknowledgment
We thank Angela Morben, DVM, ELS, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1624). TM serves as an unpaid editorial board member of Journal of Thoracic Disease from Sep 2018 to Aug 2020. TN has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Igai H, Kamiyoshihara M, Yoshikawa R, et al. Algorithm-based troubleshooting to manage bleeding during thoracoscopic anatomic pulmonary resection. J Thorac Dis. 2019;11:4544-50. [Crossref] [PubMed]
- Igai H, Kamiyoshihara M, Yoshikawa R, et al. Management of intraoperative bleeding during thoracoscopic pulmonary resection in Japan. J Vis Surg 2018;4:225. [Crossref]
- Igai H, Kamiyoshihara M, Ibe T, et al. Troubleshooting for bleeding in thoracoscopic anatomic pulmonary resection. Asian Cardiovasc Thorac Ann 2017;25:35-40. [Crossref] [PubMed]
- Miyazaki T, Sakai T, Tsuchiya T, et al. Assessment and follow-up of intercostal nerve damage after video-assisted thoracic surgery. Eur J Cardiothorac Surg 2011;39:1033-9. [Crossref] [PubMed]
- Wang Z, Pang L, Tang J, et al. Video-assisted thoracoscopic surgery versus muscle-sparing thoracotomy for non-small cell lung cancer: A systematic review and meta-analysis. BMC Surg 2019;19:144. [Crossref] [PubMed]
- Shimizu H, Okada M, Tangoku A, et al. Thoracic and cardiovascular surgeries in Japan during 2017: Annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2020;68:414-49. [Crossref] [PubMed]
- Inomata M, Shiroshita H, Uchida H, et al. Current status of endoscopic surgery in Japan: The 14th National Survey of Endoscopic Surgery by the Japan Society for Endoscopic Surgery. Asian J Endosc Surg 2020;13:7-18. [Crossref] [PubMed]
- Miyazaki T, Yamasaki N, Tsuchiya T, et al. Management of unexpected intraoperative bleeding during thoracoscopic pulmonary resection: A single institutional experience. Surg Today 2016;46:901-7. [Crossref] [PubMed]
- Yamashita S, Tokuishi K, Moroga T, et al. Totally thoracoscopic surgery and troubleshooting for bleeding in non-small cell lung cancer. Ann Thorac Surg 2013;95:994-9. [Crossref] [PubMed]
- Gonzalez-Rivas D, Stupnik T, Fernandez R, et al. Intraoperative bleeding control by uniportal video-assisted thoracoscopic surgery†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i17-24. [PubMed]
- Kocher GJ, Schmid RA, Melfi FMA. Robotic lobectomy: Tips, pitfalls and troubleshooting. Eur J Cardiothorac Surg 2014;46:e136-8. [Crossref] [PubMed]
- Liu L, Mei J, He J, et al. International expert consensus on the management of bleeding during VATS lung surgery. Ann Transl Med 2019;7:712. [Crossref] [PubMed]
- Miyazaki T, Imperatori A, Jimenez M, et al. An aggregate score to stratify the technical complexity of video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2019;28:728-34. [Crossref] [PubMed]
- Wu CF, Roel MD, Argueta AO, et al. Troubleshooting of single port video-assisted thoracoscopic lung resection. J Vis Surg 2016;2:162. [Crossref] [PubMed]
- Lucciarini P, Augustin F, Maier HT, et al. Intraoperative complications during VATS lobectomies from conversion to minimally-invasive “trouble-shooting.” J Vis Surg 2018;4:28. [Crossref] [PubMed]
- Guo C, Mei J, Ma L, et al. Handling vascular bleeding without conversion during video-assisted thoracoscopic surgery major pulmonary resection. Ann Transl Med 2018;6:363. [Crossref] [PubMed]
- Liu C, Ma L, Pu Q, et al. Troubleshooting complicated hilar anatomy via prophylactically clamping the pulmonary artery: Three videos demonstrating three techniques. Ann Transl Med 2018;6:365. [Crossref] [PubMed]