
Clinical significance of anemia as a prognostic factor in non-small cell lung cancer carcinoma with activating epidermal growth factor receptor mutations
Introduction
Anemia is a frequent finding in cancer patients. About 38% of lung cancer patients have anemia. Cancer-related anemia results from various conditions such as malnutrition, disease progression, renal or bone marrow involvement, coexisting inflammation, and oncologic treatment including chemotherapy and radiotherapy (1,2). Cancer cells can produce inflammatory cytokine such as interleukin-6 (IL-6) that can directly and indirectly lead to anemia by inhibiting erythropoiesis (3). Proinflammatory cytokine, mainly IL-6 promote alterations in erythroid progenitor proliferation, erythropoietin production and survival of circulating erythrocytes. Cancer related anemia is commonly occurred in patients at advanced disease and advancing age. Prevalence of anemia differs among cancer types, and lung cancer, gynecologic or gastrointestinal tumors are highly accompanied (4).
Pre-treatment anemia is a poor prognosis factor in various malignancies including soft tissue sarcoma, glottis larynx, pancreas, colon, breast and ovarian cancer (5-8). In lung cancer, anemia and transfusion for anemia correction are associated with poor survival after surgery of non-small cell lung cancer (NSCLC) (9). In patients with early-stage lung cancer who have undergone stereotactic body radiation therapy (SBRT), pre-treatment anemia is predictive of poor overall survival (OS) and disease progression (10). In lung cancer including NSCLC and small cell lung carcinoma, pre-treatment anemia is associated with shorter median survival time (11).
In the era of molecular targeted therapy, epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) has shown remarkable outcomes with minimal side effects compared to conventional chemotherapy (12). In locally advanced NSCLC, especially patients with old age or poor performance status (PS), EGFR-TKI has shown similar efficacy comparable to chemotherapy, the standard treatment (13).
Multiple prognostic factors including sex, histology, clinical stage, the presence of pleural effusion or brain metastasis, PS, body mass index (BMI) and prognostic nutritional index have been evaluated in NSCLC patients with activating EGFR mutations (14). Although pre-treatment anemia is a known prognostic marker for survival and clinical outcomes of patients treated with cytotoxic chemotherapy, surgery, or SBRT, few studies have been conducted in NSCLC patients treated with EGFR-TKI. Thus, the objective of this study was to investigate the prognostic value of pre-treatment anemia in NSCLC patients with activating EGFR mutations treated with EGFR-TKI.
Methods
This was a multicenter retrospective study conducted in seven hospitals of the Catholic Medical Center in South Korea: Seoul St. Mary’s Hospital, Yeouido St. Mary’s Hospital, Eunpyeong St. Mary’s Hospital, Bucheon St. Mary’s Hospital, Uijeongbu St. Mary’s Hospital, St. Vincent’s Hospital, and Incheon St. Mary’s Hospital. All data were collected from hospital database.
Patients
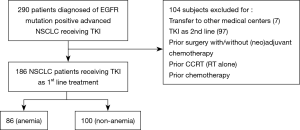
Patients were eligible if they were diagnosed from January 2009 to February 2016 with histologically confirmed, primary NSCLC [International Classification of Diseases for Oncology (ICD): C34.X] and EGFR mutation positive advanced NSCLC treated at our institutions. Use of TKI was investigated using drug codes for iressa, gefitinib, or afatinib during study period. Patients who were transferred to other centers or treated with EGFR-TKI as 2nd line were excluded. The study flow is summarized in Figure 1. The follow-up period ended on December 31, 2017.
Data
We extracted the following data from patient medical records: patient demographics, smoking history, stage of lung cancer, comorbid diseases, Eastern Cooperative Oncology Group (ECOG) PS, laboratory data, history of chemotherapy and/or radiation, survival status, and dates of disease progression and death. Blood samples drawn to evaluate hemoglobin level within one month before EGFR-TKI treatment were used to compile a pretreatment hematologic profile for each patient using laboratory value most proximal to EGFR-TKI treatment. Pre-treatment anemia was defined according to World Health Organization (WHO) criteria (Hb concentration <13 g/dL for men and <12 g/dL for women) and tested before EGFR TKI treatment in a month (15). Anemia was divided into mild and moderate anemia according to the scoring system provided by the National Cancer Institute and the National Institutes of Health: mild anemia, hemoglobin level of 10.0–11.9 g/dL for women and 10.0–12.9 g/dL for men; moderate anemia, hemoglobin level of 8.0–9.9 g/dL (16). Simplified Comorbidity Score (SCS) comorbidity items were defined according to Colinet et al. (17). Items of SCS and their weighing based on risk of death resulted in seven comorbidity groupings as follows: tobacco consumption (weighing: 7), diabetes mellitus (weighing: 5), renal insufficiency (weighing: 4), respiratory comorbidity (weighing: 1), cardiovascular comorbidity (weighing: 1), neoplastic comorbidity (weighing: 1), and alcoholism (weighing: 1).
Statistical analysis
Baseline demographics and clinical outcomes were compared between patients with anemia and non-anemia. We used Pearson’s chi-square test to compare discrete variables and Student’s t-test or analysis of variance to compare continuous variables. The Mann-Whitney test was used to compare median value. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were calculated for predictors that were significant in multivariate Cox regression analysis. A two-sided P value <0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS for Windows software (ver. 20.0; IBM Corp., Armonk, NY, USA).
Results
Overall, 290 patients were diagnosed with NSCLC harboring activating EGFR mutations and treated with EGFR-TKI, of whom 104 met the exclusion criteria (transferred to another medical center or treated with EGFR-TKI as 2nd line treatment). Thus, 186 patients were finally included in our analysis (Figure 1). The mean age of included subjects was 67.51±11.65 years (range, 39–97 years). There were 64 (34.4%) males. Of all included patients, 86 (46.2%) and 100 (53.8%) were classified into anemia and non-anemia groups, respectively. In anemia patients, 15 (8.1%) had moderate and 69 (38.2%) had mild severity of anemia based on the scoring system of the National Cancer Institute and the National Institutes of Health. In patients with anemia, we compared these two groups and explored clinical factors predicting OS. Total and median follow-up time were 19.4 and 15.2 person-months, respectively.
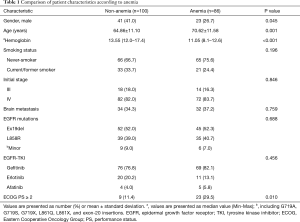
Baseline characteristics of these two groups are summarized in Table 1. The proportion of male was lower in the anemia group (26.7% vs. 41.0%, P=0.045). The rate of poor PS (≥2) based on ECOG PS was higher in the anemia group (29.5% vs. 11.4%, P=0.010). Mean age was higher in the anemia group (70.62±11.58 vs. 64.86±11.10 years, P<0.001). Smoking history, cancer staging, and sensitizing EGFR expressions showed no significant differences between the two groups.
Full table
Patients with anemia group displayed a shorter median OS than those with non-anemia [24.83 (95% CI, 17.49–32.17) months vs. 42.10 (95% CI, 31.87–52.34) months, P=0.031)]. However, there was no difference in progression free survival (PFS) between anemia and non-anemia group [10.27 (95% CI, 7.98–12.55) months vs. 10.93 (95% CI, 9.14–12.73 months, P=0.865).When the patients with anemia group were divided into two subgroups, mild and moderate anemia based on the severity of anemia, moderate anemia group had the shortest OS among the three groups (10.03±3.82 vs. 27.23±3.56 vs. 42.10±4.89 months, P=0.001) (Figure 2).

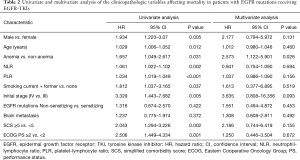
In univariate analysis for OS, HR was 1.934 (95% CI, 1.220–3.07, P=0.005) for male, 1.029 (95% CI, 1.006–1.052, P=0.012) for older age, 1.657 (95% CI, 1.049–2.617, P=0.031) for anemia, 1.061 (95% CI, 1.022-1.102, P=0.002) for higher NLR, 1.034 (95% CI, 1.019–1.049) for higher PLR, 1.812 (95% CI, 1.037–3.165, P=0.037) for smoking status, 3.329 (95% CI, 1.443–7.682, P=0.005) for stage IV compared to stage III, 2.043(95% CI, 1.294–3.226, P=0.002) for SCS equal to or higher than 5, and 2.506 (95% CI, 1.449–4.334, P=0.001) for poor PS. In multivariate analysis, anemia (aHR, 2.573; 95% CI, 1.122–5.901; P=0.026) was only independent factors for poor OS (Table 2).
Full table
Discussion
We compared clinical outcomes of patients with NSCLC harboring activating EGFR mutations treated with EGFR-TKI based on hemoglobin levels and explored prognostic clinical factors for treatment outcomes. In the present study, we found that 46.2% of 186 cancer patients had anemia at the time of diagnosis. We also found that the proportion of male was lower in the anemia group while poor PS and mean age were higher in the anemia group. Patients with anemia displayed shorter median OS than those with non-anemia. When patients with anemia were divided into mild and moderate anemia subgroups based on the severity of anemia, the moderate anemia group had the shortest OS among the three groups. In multivariate analysis, anemia was only independent factor for poor OS.
Anemia is a common hematological manifestation of cancer (1,2,18). Anemia is considered a side effect associated with chemotherapy. However, many cancer patients already have anemia before the start of any treatment (18). Pre-treatment anemia can negatively influence clinical outcomes in various types of malignancies including soft tissue sarcoma, pancreatic cancer and ovarian carcinoma (19,20). Low hemoglobin contributes to inadequate oxygenation of squamous cell carcinoma of the head and neck and increased resistance to radiotherapy (21). In lung cancer, anemia at diagnosis of the disease is associated with reduced median survival time (11). Pre-treatment anemia is associated with poor survival and non-local disease progression in patients with SBRT (10). Peri-operative anemia and blood transfusion for anemia correction are also associated with shorter recurrence free survival and OS after NSCLC surgery (9).
Known clinical factors affecting prognosis are sex, histology, clinical stage, the presence of pleural effusion, and PS in NSCLC with EGFR mutations receiving EGFR-TKI as 1st line treatment (22). In NSCLC patients with EGFR mutations receiving EGFR-TKI regardless of the order of treatment, ECOG PS, brain metastasis, low BMI, and prognostic nutritional index are poor prognostic factors for OS (14). In EGFR-mutant NSCLC patients who are re-administered TKIs after failure of first-line TKIs, systemic inflammatory status in terms of neutrophil-to-lymphocyte ratio and lymphocyte-to-monocyte ratio are prognostic factors (23). However, little data exist about the prognostic role of anemia in NSCLC patients with EGFR mutations treated with EGFR-TKI.
There are many causes for anemia. It involves nutritional status and other comorbidities. A direct link between anemia and poor prognosis in NSCLC patients is difficult to explain. One possible mechanism is inflammatory cytokine IL-6. IL-6 is a cytokine that plays an important role in many chronic inflammatory diseases. Most NSCLC cell lines produce IL-6 (3). IL-6 expression is involved in the regulation of tumor growth and metastasis. Activation of the IL-6R/JAK1/STAT3 pathway is known to induce resistance to irreversible EGFR-TKI in NSCLC harboring T790M (24). IL-6 is also a potent inducer for the production of hepcidin, a key regulator of iron metabolism (25). Elevation of hepcidin can lead to reduction in iron levels and results in anemia (26). Higher hepcidin levels are also associated with more aggressive disease (27).
Angiogenesis is a major prognostic factor in cancer. It is regulated by vascular endothelial growth factor (VEGF). Clinically, cancer patients with elevated levels of VEGF have worse prognosis compared to patients with normal VEGF levels (28). Tumor hypoxia and serum concentration of VEGF are strongly correlated with each other, presenting a main stimulus for angiogenesis in tumors (29). Also, anemia and poor tissue oxygenation are associated in head and neck cancers (21). In patients with untreated solid cancers of various malignancies including head and neck, cervix, rectum, and lung cancers, anemic patients have elevated levels of VEGF (30).
EGFR and VEGF signaling pathways are closely connected to each other. The expression of VEGF is up-regulated by EGFR phosphorylation (31). EGFR-TKI resistance is associated with increased expression of VEGF. A combination of VEGF inhibitor bevacizumab with EGFR-TKI can abrogate primary resistance and demonstrate substantial antitumor activity (32). Although our study did not investigate the association between anemia and VEGF expression, it might be one of mechanisms that can explain the poor prognosis in the anemia group.
Limitations
This study has some limitations. First, it was a retrospective study. Nonetheless, our study was a multi-center study having a moderate sample size. Second, the proportion of poor PS was higher in the anemia group. Cancer-related anemia results from multiple causes such as malnutrition, deteriorated intestinal absorption and metabolism, chronic inflammation, aging, and advanced stage, which are factors for decreased erythropoiesis (33). In addition to anemia, aging and malnutrition might contribute to poor prognosis. However, after adjusting for age and poor PS, anemia was an independent prognostic factor in NSCLC with EGFR mutations treated with TKI.
Conclusions
Findings of this study showed that pre-treatment anemia is significantly associated with decreased OS in NSCLC patients with EGFR mutations treated with EGFR-TKI. Our data indicate that hemoglobin level routinely measured during cancer work up may represent a potentially important variable to predict prognosis in NSCLC patients harboring activating EGFR mutations treated with EGFR-TKI. Further large-scale prospective studies are required to validate our findings.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-3932). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by institutional review boards (IRBs) of all participating centers. The requirement for informed consent was waived by IRBs because the study was based on retrospective chart reviews (IRB No HC19RCDI0027).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lang E, Bissinger R, Qadri SM, et al. Suicidal death of erythrocytes in cancer and its chemotherapy: A potential target in the treatment of tumor-associated anemia. Int J Cancer 2017;141:1522-8. [Crossref] [PubMed]
- Zabłocka-Słowińska KA, Kosacka M, Porebska I, et al. The usefulness of routinely used malnutrition screening tools in predicting anemia in lung cancer patients. Adv Clin Exp Med 2017;26:1383-9. [Crossref] [PubMed]
- Yamaji H, Iizasa T, Koh E, et al. Correlation between interleukin 6 production and tumor proliferation in non-small cell lung cancer. Cancer Immunol Immunother 2004;53:786-92. [Crossref] [PubMed]
- Madeddu C, Gramignano G, Astara G, et al. Pathogenesis and Treatment Options of Cancer Related Anemia: Perspective for a Targeted Mechanism-Based Approach. Front Physiol 2018;9:1294. [Crossref] [PubMed]
- Lutterbach J, Guttenberger R. Anemia is associated with decreased local control of surgically treated squamous cell carcinomas of the glottic larynx. Int J Radiat Oncol Biol Phys 2000;48:1345-50. [Crossref] [PubMed]
- An MS, Yoo JH, Kim KH, et al. T4 stage and preoperative anemia as prognostic factors for the patients with colon cancer treated with adjuvant FOLFOX chemotherapy. World J Surg Oncol 2015;13:64. [Crossref] [PubMed]
- Obermair A, Handisurya A, Kaider A, et al. The relationship of pretreatment serum hemoglobin level to the survival of epithelial ovarian carcinoma patients: a prospective review. Cancer 1998;83:726-31. [Crossref] [PubMed]
- Zhang Y, Chen Y, Chen D, et al. Impact of preoperative anemia on relapse and survival in breast cancer patients. BMC Cancer 2014;14:844. [Crossref] [PubMed]
- Cata JP, Gutierrez C, Mehran RJ, et al. Preoperative anemia, blood transfusion, and neutrophil-to-lymphocyte ratio in patients with stage i non-small cell lung cancer. Cancer Cell Microenviron 2016;3:e1116. [PubMed]
- Shaverdian N, Veruttipong D, Wang J, et al. Pretreatment Anemia Portends Poor Survival and Nonlocal Disease Progression in Patients with Stage I Non-Small Cell Lung Cancer Treated with Stereotactic Body Radiation Therapy. J Thorac Oncol 2016;11:1319-25. [Crossref] [PubMed]
- Aoe K, Hiraki A, Maeda T, et al. Serum hemoglobin level determined at the first presentation is a poor prognostic indicator in patients with lung cancer. Intern Med 2005;44:800-4. [Crossref] [PubMed]
- Yang JC, Hirsh V, Schuler M, et al. Symptom control and quality of life in LUX-Lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3342-50. [Crossref] [PubMed]
- Hsia TC, Liang JA, Li CC, et al. Comparative effectiveness of concurrent chemoradiotherapy versus EGFR-tyrosine kinase inhibitors for the treatment of clinical stage IIIb lung adenocarcinoma patients with mutant EGFR. Thorac Cancer 2018;9:1398-405. [Crossref] [PubMed]
- Lin JH, Lin D, Xu L, et al. The association between clinical prognostic factors and epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) efficacy in advanced non-small-cell lung cancer patients: a retrospective assessment of 94 cases with EGFR mutations. Oncotarget 2017;8:3412-21. [Crossref] [PubMed]
- Balducci L. Anemia, cancer, and aging. Cancer Control 2003;10:478-86. [Crossref] [PubMed]
- Experts Committee on Cancer -Related Anemia, Chinese Society of Clinical Oncology (CSCO). Clinical practice guidelines on cancer-related anemia (2012-2013 Edition). Chin Clin Oncol 2012;1:18. [PubMed]
- Colinet B, Jacot W, Bertrand D, et al. A new simplified comorbidity score as a prognostic factor in non-small-cell lung cancer patients: description and comparison with the Charlson's index. Br J Cancer 2005;93:1098-105. [Crossref] [PubMed]
- Macciò A, Madeddu C, Gramignano G, et al. The role of inflammation, iron, and nutritional status in cancer-related anemia: results of a large, prospective, observational study. Haematologica 2015;100:124-32. [Crossref] [PubMed]
- Ruiz-Tovar J, Martin-Perez E, Fernandez-Contreras ME, et al. Identification of prognostic factors in pancreatic cancer. Cir Cir 2011;79:313-22. [PubMed]
- Szkandera J, Gerger A, Liegl-Atzwanger B, et al. Pre-treatment anemia is a poor prognostic factor in soft tissue sarcoma patients. PLoS One 2014;9:e107297. [Crossref] [PubMed]
- Becker A, Stadler P, Lavey RS, et al. Severe anemia is associated with poor tumor oxygenation in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys 2000;46:459-66. [Crossref] [PubMed]
- Inoue A, Yoshida K, Morita S, et al. Characteristics and overall survival of EGFR mutation-positive non-small cell lung cancer treated with EGFR tyrosine kinase inhibitors: a retrospective analysis for 1660 Japanese patients. Jpn J Clin Oncol 2016;46:462-7. [Crossref] [PubMed]
- Chen YM, Lai CH, Rau KM, et al. Impact of clinical parameters and systemic inflammatory status on epidermal growth factor receptor-mutant non-small cell lung cancer patients readministration with epidermal growth factor receptor tyrosine kinase inhibitors. BMC Cancer 2016;16:868. [Crossref] [PubMed]
- Huang L, Fu L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B 2015;5:390-401. [Crossref] [PubMed]
- Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006;108:3204-9. [Crossref] [PubMed]
- Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003;102:783-8. [Crossref] [PubMed]
- Chen Q, Wang L, Ma Y, et al. Increased hepcidin expression in non-small cell lung cancer tissue and serum is associated with clinical stage. Thorac Cancer 2014;5:14-24. [Crossref] [PubMed]
- Linderholm B, Grankvist K, Wilking N, et al. Correlation of vascular endothelial growth factor content with recurrences, survival, and first relapse site in primary node-positive breast carcinoma after adjuvant treatment. J Clin Oncol 2000;18:1423-31. [Crossref] [PubMed]
- Dunst J, Stadler P, Becker A, et al. Tumor hypoxia and systemic levels of vascular endothelial growth factor (VEGF) in head and neck cancers. Strahlenther Onkol 2001;177:469-73. [Crossref] [PubMed]
- Dunst J, Becker A, Lautenschlager C, et al. Anemia and elevated systemic levels of vascular endothelial growth factor (VEGF). Strahlenther Onkol 2002;178:436-41. [Crossref] [PubMed]
- Li F, Zhu T, Cao B, et al. Apatinib enhances antitumour activity of EGFR-TKIs in non-small cell lung cancer with EGFR-TKI resistance. Eur J Cancer 2017;84:184-92. [Crossref] [PubMed]
- Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387-401. [Crossref] [PubMed]
- Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer-related anemia. Am J Hematol 2014;89:203-12. [Crossref] [PubMed]


