Prognostic nutritional index as a predictor of mortality in nontuberculous mycobacterial lung disease
Introduction
The prevalence of nontuberculous mycobacterial lung disease (NTM-LD) is increasing every year (1). Malnutrition is often found in advanced NTM-LD (2). The prognostic nutritional index (PNI) and Glasgow Prognostic Score (GPS) are known as indicators related to nutrition. In tuberculosis, the serum albumin level and PNI score are associated with the cavitation (3). Moreover, the GPS, which is a prognostic indicator for malignant tumors, predicts the prognosis of idiopathic pulmonary fibrosis (4), but there are no reports on the association between these indicators and NTM-LD. We statistically analyzed the association between these indicators and the prognosis of NTM-LD.
Methods
Subjects
This was a single-center retrospective study. Among the patients who visited the Department of Respiratory Medicine at the Maebashi Red Cross Hospital from January 1, 2014, to December 31, 2018, those who were diagnosed according to the criteria of the American Thoracic Society/Infectious Disease Society of America from 2007 were enrolled (5). Patients lacking information, patients less than 20 old years, and patients who refused to enroll in the trial were excluded. Patients diagnosed with NTM were followed up until the last visit of the study period. This study disclosed information using an opt-out method and excluded patients who did not consent to the study. This study was reviewed and approved by the Maebashi Red Cross Hospital Ethics Committee on July 5, 2019 (acceptance no. 2019–15). The outcome of this study did not affect the future, and the personal data of the subject was strictly secured.
Clinical assessment
Statistical data (date, height, and weight at the time of NTM-LD diagnosis and sex), sociological data (underlying diseases, smoking history), hematologic data at the time of NTM-LD diagnosis, bacteriologic data, and imaging data were acquired. Body mass index (BMI), PNI [10 × serum albumin level (g/dL) + 0.005 × lymphocyte count (/µL)] (6), and GPS were calculated from the acquired data. The GPS was defined as 2 points, 1 point, and 0 point with elevated C-reactive protein (CRP) (>1 mg/dL) and hypoalbuminemia (<3.5 g/dL), elevated CRP (>1 mg/dL) or hypoalbuminemia (<3.5 g/dL), and neither elevated CRP nor hypoalbuminemia, respectively (7). We categorized the patients based on the onset of malignant tumors during the follow-up as follows: the none group, patients without tumor development; the past group, patients with a tumor diagnosed more than 1 month before the NTM-LD diagnosis; the concurrent group, patients with a tumor diagnosed within 1 month before or after the NTM-LD diagnosis; the future group, patients with a tumor diagnosed more than 1 month after the NTM-LD diagnosis. Also, we defined the death group as the patients who died during the follow-up.
Statistical analysis
Statistical analysis was performed using the software R version 3.4.1 and EZR version 1.37. Continuous variables were analyzed using the Mann-Whitney U test and expressed as the median (maximum and minimum). Nominal variables were analyzed using Fisher’s exact test and expressed as the number and ratio. P value <0.05 was considered statistically significant.
A logistic regression analysis was performed for items with a significant difference, and the odds ratio and 95% confidence interval were calculated. A multivariate analysis was performed after adjusting for age and sex.
Statistical analysis limited to Mycobacterium avium complex (MAC) patients was performed similarly.
Results
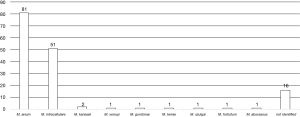
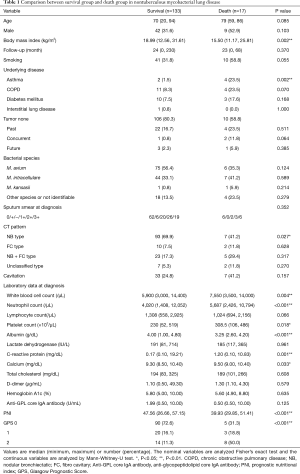
Two hundred and fifteen patients visited our hospital as NTM in the study period, excluding 60 patients who did not meet the American Thoracic Society/Infectious Disease Society of America criteria and 5 patients who were diagnosed with non-NTM. We enrolled 150 patients with NTM-LD who visited our hospital from January 2014 to December 2018. The median follow-up time was 24 months (range, 0–230 months), and 17 patients died during the follow-up time. No patients died by NTM-LD. The presence of symptoms before the study period could not investigate because of the lack of information. The median age at the time of diagnosis was 70.0 years (range, 20–94 years). There were 51 men (34.0%) and 99 women (66.0%). No patients in this study were positive for the human immunodeficiency virus. Among the identified bacterial species, M. avium, M. intracellulare, and co-infection with M. avium and M. intracellulare accounted for 81 (54.0%), 51 (34.0%), and 6 (4.0%), respectively (Figure 1). In addition, M. kansasii, M. xenopi, M. gordonae, M. terrae, M. szulgai, M. fortuitum, and M. abscessus were identified. The bacterial species were unidentified in 16 cases because of the denaturation of the bacteria or the lack of the examination by DNA-DNA hybridization method. We divided the patients into the survival and death groups and analyzed the factors associated with death (Table 1). In the death group, patients were older at the time of diagnosis {79 [59–86] vs. 70 [20–94] years; P=0.085}, and there was a higher proportion of male [9 (52.9) vs. 42 (31.6), P=0.103], but without a significant difference. BMI was significantly lower in the death group [15.50 (11.17–25.81) vs. 18.99 (12.56–31.61) (kg/m2); P=0.002], and there were significantly more cases with asthma [4 (23.5) vs. 2 (1.5); P=0.002]. Computed tomography (CT) at the first visit showed significantly fewer cases of nodular bronchiectasis (NB) type in the death group [7 (41.2) vs. 93 (69.9); P=0.027]. There was no difference cavitation, the bacterial species or the degree of sputum smear between the survival and death groups. In addition, we analyzed the association between tumor complications and death before the diagnosis, at the time of diagnosis, and after the diagnosis but found no significant differences. Hematologic examination at the time of NTM-LD diagnosis revealed that the death group had significantly higher white blood cell count [7,550 (3,500–14,000) vs. 5,900 (3,000–14,400) (/µL); P=0.004], neutrophil count [5,687 (2,426–10,794) vs. 4,020 (1,408 vs. 12,052) (/µL); P<0.001), and platelet count [308.5 (106–486) vs. 230.0 (52–519) (×103/µL); P=0.018]. The serum albumin level was significantly lower in the death group [3.25 (2.60–4.20) vs. 4.00 (1.00–4.80) (g/dL); P<0.001), while the CRP level [1.20 (0.10–10.83) vs. 0.17 (0.1–19.21) (mg/dL); P=0.001] and calcium concentration [9.50 (9.00–10.40) vs. 9.30 (8.50–10.40) (mg/dL); P=0.033] were significantly higher. The anti-glycopeptidolipid core IgA antibody was higher in the survival group, although without statistical significance [1.99 (0.50–10.00) vs. 0.5 (0.5–10.0) (U/mL); P=0.125]. PNI was significantly lower in the death group [39.93 (29.85–51.41) vs. 47.56 (26.66–57.15); P<0.001], while GPS was significantly higher (P<0.001).
Full table
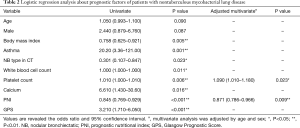
The logistic regression analysis was performed for the items with P<0.05 (BMI, presence of asthma, NB type on CT, white blood cell and platelet counts, calcium concentration, PNI, and GPS) (Table 2). The serum albumin and CRP levels and neutrophil count were excluded based on collinearity. In the multivariate analysis after adjusting for age and sex, the platelet count [odds ratio (OR): 1.090; 95% confidence interval (CI): 1.010–1.180; P=0.023] and PNI (OR: 0.871; 95% CI: 0.786–0.966; P=0.009) were the independent factors predicting prognosis.
Full table
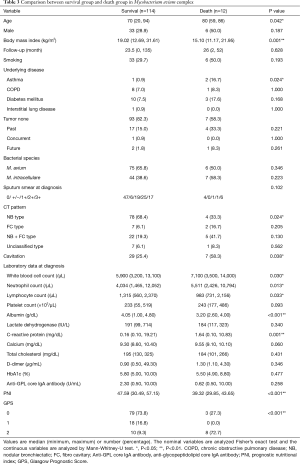
We added the analysis against MAC (Table 3). Similar to all NTM species, we found significant differences in BMI, asthma, NB type in CT, WBC, Neutrophil count, Albumin, CRP, PNI, and GPS. In addition, significant differences were found in age, lymphocyte count, and cavitation, but no significant differences were found in serum calcium value and platelet count. Logistic regression analysis was performed for items with significant differences (Table 4). In a multivariate analysis corrected for age and sex, BMI and GPS were independent predictors of death.
Full table

Full table
Discussion
NTM-LD has a high mortality rate. Marras et al. reported mortality rates of 20.7 per 1,000 person-years and 5.6 per 1,000 person-years in the NTM-LD and control groups, respectively (8). In addition, pulmonary hypertension, pulmonary fibrosis, male sex, older age, and immunosuppressive therapy use are risk factors for high mortality in patients NTM-LD (9,10). Although there are a few reports on the association between malignant tumors and NTM-LD, lung cancer was present in 1.7–3.9% of patients with NTM-LD (11,12).
In our knowledge, this is the first report that PNI was identified as an independent factor predicting death. The PNI score is calculated using the lymphocyte count and serum total cholesterol level (6). Initially, it was reported that low PNI scores were associated with the prognosis of gastrointestinal cancers after surgery. Additionally, recent reports stated that PNI predicted the development of cavitation in pulmonary tuberculosis (3) and was associated with one-year mortality in heart failure (13). Although it is unclear why PNI is associated with death in NTM-LD, it may be because the decline in nutritional and immunologic statuses is associated with the development and progression of secondary diseases in addition to the progression of NTM-LD. The sputum smear at the first visit or the NTM species were not associated with death, and the association between the NTM-LD disease status and death was not clear. The lymphocyte count and serum total cholesterol level in PNI were not significantly associated with death in this study, but it was reported that low lymphocyte counts predicted three-year mortality in severe chronic obstructive pulmonary disease (14), and the absolute lymphocyte count was an independent predictor of long-term mortality in acute heart failure (15). It is also known that hypocholesterolemia is associated with the development of some cancers (16). Low lymphocyte counts and hypocholesterolemia are associated with death in other diseases, and their combination might predict the death.
We examined GPS as a measure of the nutritional status, but it was not an independent factor associated with death. GPS is calculated from the CRP and serum albumin levels and predicts the prognosis in non-small cell lung cancer (7). Cowman et al. divided NTM-LD into the cavitary, nodular, and bronchiectasis types and found that lower albumin and higher CRP levels were associated with poor prognosis of cavitary NTM-LD (17). It is unclear why GPS based on the CRP and serum albumin levels is not associated with mortality in NTM-LD. One possibility is that NTM-LD needs different cut-off values when scoring CRP and serum albumin levels; however, this hypothesis has not been proven. On the other hand, GPS was an independent factor predicting death in the MAC group. Previous papers have reported that BMI predicted death in MAC (18,19), and in this study, BMI predicted death in the MAC group, but not all NTM. Although some predicted factors in mortality reported in the past were consistent with our study, some factors were not. As well, the different result was found in all NTM species and the MAC group. The differences in these results are difficult to explain but one of the reasons may be for the small number of patients included in this study. It is difficult to guess with only this study, and additional research with an increased number of patients is needed.
This study suggested that high platelet count at the time of NTM diagnosis may predict the death. In tuberculosis, it has been reported that the platelet associated with increased disease activity, proinflammatory, and tissue destruction through platelet-associated mediators (20,21). Although the role of platelets in NTM are not known in detail, the platelets may be associated with inflammatory by a mechanism similar to tuberculosis.
There were more patients based on asthma in the death group. There are reports that the use of inhaled corticosteroid (ICS) is involved in the development of NTM-LD (22,23). We cannot discuss the relationship between ICS use and death because we have not investigated the treatment of asthma, but NTM-LD may require careful use of ICS.
There are several limitations to this study. First, this was a single-center retrospective study with small sample size. In this study, only 150 people were enrolled, and the power to perform various analyzes is limited. Although it has been reported that age and sex are associated with death in NTM (9,10), our study did not show a significant difference. The result of P=0.090 for age and P=0.087 for sex in the univariate logistic analysis was not significantly but tend to associate. Analysis of the MAC group provided similar results to previously reported predictors of mortality, and the analysis for NTM may also be reliable. The little number of the patient may be the reason for the lack of significant results, and the study that increased enrolled patients is considered necessary for further studies. Second, the follow-up duration was relatively short and might not have been sufficient to examine the association of the parameters with death. Third, the bacterial species was not identified in some cases because of the denaturation of the bacteria or lack of examination; therefore, the data might not be homogeneous in the background factors. Finally, the analysis was performed using hematologic data at the time of diagnosis and could not be performed after the diagnosis because of the short follow-up duration and the lack of homogenized data. There is a need to examine whether or not the platelet count or PNI after the diagnosis could predict the prognosis. Future prospective multi-center studies with more cases and a longer follow-up duration are required.
Conclusions
Our data indicated that the PNI and platelet count at the time of NTM-LD diagnosis might be associated with mortality. Prospective, large-scale, multi-center studies are warranted.
Acknowledgments
We thank Editage staff for checking the grammar and proofreading. We are also grateful to the Maebashi Red Cross Hospital staff for helping us with the medical care and data collection.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-803). Dr. Horie reports personal fees from Astra Zeneca, personal fees from Teijin Pharma, personal fees from Boehringer Ingelheim Japan, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study disclosed information using an opt-out method and excluded patients who did not consent to the study. This study was reviewed and approved by the Maebashi Red Cross Hospital Ethics Committee on July 5, 2019 (acceptance no. 2019–15).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med 2015;36:13-34. [Crossref] [PubMed]
- Morimoto K, Yoshiyama T, Kurashima A, et al. Nutritional indicators are correlated with the radiological severity score in patients with Mycobacterium avium complex pulmonary disease: a cross-sectional study. Intern Med 2014;53:397-401. [Crossref] [PubMed]
- Nakao M, Muramatsu H, Arakawa S, et al. Immunonutritional status and pulmonary cavitation in patients with tuberculosis: A revisit with an assessment of neutrophil/lymphocyte ratio. Respir Investig 2019;57:60-6. [Crossref] [PubMed]
- Kang HS, Cho KW, Kwon SS, et al. Prognostic significance of Glasgow prognostic score in patients with acute exacerbation of idiopathic pulmonary fibrosis. Respirology 2018;23:206-12. [Crossref] [PubMed]
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367-416. [Crossref] [PubMed]
- Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984;85:1001-5. [PubMed]
- Forrest LM, McMillan DC, McArdle CS, et al. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer 2003;89:1028-30. [Crossref] [PubMed]
- Marras TK, Vinnard C, Zhang Q, et al. Relative risk of all-cause mortality in patients with nontuberculous mycobacterial lung disease in a US managed care population. Respir Med 2018;145:80-8. [Crossref] [PubMed]
- Fleshner M, Olivier KN, Shaw PA, et al. Mortality among patients with pulmonary non-tuberculous mycobacteria disease. Int J Tuberc Lung Dis 2016;20:582-7. [Crossref] [PubMed]
- Novosad SA, Henkle E, Schafer S, et al. Mortality after Respiratory Isolation of Nontuberculous Mycobacteria. A Comparison of Patients Who Did and Did Not Meet Disease Criteria. Ann Am Thorac Soc 2017;14:1112-9. [PubMed]
- Tsuji T, Tsuyuguchi K, Tachibana K, et al. Analysis of the impact of lung cancer treatment on nontuberculous mycobacterial lung diseases. Respir Investig 2017;55:45-50. [Crossref] [PubMed]
- Kusumoto T, Asakura T, Suzuki S, et al. Development of lung cancer in patients with nontuberculous mycobacterial lung disease. Respir Investig 2019;57:157-64. [Crossref] [PubMed]
- Shirakabe A, Hata N, Kobayashi N, et al. The prognostic impact of malnutrition in patients with severely decompensated acute heart failure, as assessed using the Prognostic Nutritional Index (PNI) and Controlling Nutritional Status (CONUT) score. Heart Vessels 2018;33:134-44. [Crossref] [PubMed]
- Acanfora D, Scicchitano P, Carone M, et al. Relative lymphocyte count as an indicator of 3-year mortality in elderly people with severe COPD. BMC Pulm Med 2018;18:116. [Crossref] [PubMed]
- Carubelli V, Bonadei I, Castrini AI, et al. Prognostic value of the absolute lymphocyte count in patients admitted for acute heart failure. J Cardiovasc Med (Hagerstown) 2017;18:859-65. [Crossref] [PubMed]
- Iso H, Ikeda A, Inoue M, et al. Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. Int J Cancer 2009;125:2679-86. [Crossref] [PubMed]
- Cowman SA, Jacob J, Obaidee S, et al. Latent class analysis to define radiological subgroups in pulmonary nontuberculous mycobacterial disease. BMC Pulm Med 2018;18:145. [Crossref] [PubMed]
- Gochi M, Takayanagi N, Kanauchi T, et al. Retrospective study of the predictors of mortality and radiographic deterioration in 782 patients with nodular/bronchiectatic Mycobacterium avium complex lung disease. BMJ Open 2015;5:e008058. [Crossref] [PubMed]
- Kumagai S, Ito A, Hashimoto T, et al. Development and validation of a prognostic scoring model for Mycobacterium avium complex lung disease: an observational cohort study. BMC Infect Dis 2017;17:436. [Crossref] [PubMed]
- Kassa E, Enawgaw B, Gelaw A, et al. Effect of anti-tuberculosis drugs on hematological profiles of tuberculosis patients attending at University of Gondar Hospital, Northwest Ethiopia. BMC Hematol 2016;16:1. [Crossref] [PubMed]
- Fox KA, Kirwan DE, Whittington AM, et al. Platelets Regulate Pulmonary Inflammation and Tissue Destruction in Tuberculosis. Am J Respir Crit Care Med 2018;198:245-55. [Crossref] [PubMed]
- Brode SK, Campitelli MA, Kwong JC, et al. The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur Respir J 2017. [Crossref] [PubMed]
- Liu VX, Winthrop KL, Lu Y, et al. Association between Inhaled Corticosteroid Use and Pulmonary Nontuberculous Mycobacterial Infection. Ann Am Thorac Soc 2018;15:1169-76. [Crossref] [PubMed]