
Clinical efficacy of robot-assisted thoracoscopic surgery for posterior mediastinal neurogenic tumors
Introduction
Tumors of the posterior mediastinum are usually diagnosed in young patients, but they can develop at any age and form any tissue that exists in or passes through the chest cavity (1,2). The diagnosis and the accurate assessment of the posterior mediastinum masses represent a challenge, given their clinical variability from asymptomatic to producing symptoms of cough, chest pain, and dyspnea. Posterior Mediastinal tumors are often neurogenic tumors and esophageal cysts. The neurogenic tumors are mainly composed of schwannoma, neurofibroma and ganglioneuroma (2-4). The traditional open transthoracic procedure is the standard surgery for posterior mediastinal neurogenic tumor (PMNT) (5,6). However, the video-assisted thoracoscopic surgery (VATS), with less surgical trauma, blood loss, postoperative complications, and shorter hospital stay, has been gradually recognized as a more feasible surgical procedure for posterior mediastinal neurogenic tumor (6-8). Also, the clinical outcomes of video-assisted thoracoscopic surgery were found to be more effective and safe than standard open surgery (9,10). However, the VATS is limited by a two-dimensional view and the reduced freedom of movements, which may disable delicate dissections of the posterior mediastinal neurogenic tumor, especially when the tumor was at a superior intrathoracic position. With the recent developments in surgical instruments, robot-assisted thoracic surgery (RATS) performing with the Da Vinci Si robotic system has an increasing usage throughout the world (11-13). Comparing with VATS, RATS offers superiorities, in terms of tremor filtration, three-dimensional visualization, ten-times-enlarged image and seven-degree freedom of its dexterity endowrists, which enable the surgeon to operate in a stable and comfortable environment. RATS has already widely used in esophagectomy, lobectomy and other minimally invasive thoracic surgeries in recent years (14-17). Nonetheless, whether the RATS is suitable for resection of posterior mediastinal neurogenic tumor is still unclear. Only few case reports were recorded to show the advantage of RATS in PMNT.
Methods
We retrospectively reviewed the clinical data of 130 patients diagnosed with posterior mediastinal neurogenic tumor between 2015 and 2018 at the Department of Thoracic Surgery in Nanjing Jinling Hospital. The diagnoses and clinical information were collected by consulting medical records of the patients. Magnetic resonance imaging (MRI) or enhanced computed tomography scan (CT-scan) was used to locate the tumor and investigate the Adamkiewicz’s artery preoperatively. Patient inclusion criteria were as follows: (I) diagnosis with resectable PMNT with tumor sizes less than 8 cm and (II) willingness to undergo a robot-assisted operation or video-assisted operation. And the excluded criteria were as follows: patients with cardiomegaly, poor cardiac function or severe arrhythmia. The clinical characteristics were composed of sex, age, body mass index (BMI), smoking and comorbidity (including chronic obstructive pulmonary disease (COPD), hypertension and diabetes. Tumor associated data, in terms of tumor size and tumor types, were also collected. The operative and postoperative data included surgical time, blood loss, conversion rate, duration of chest tube insertion, median volume of drainage, postoperative hospital stay, postoperative complications and adverse reactions. All patients involved in this study gave their informed consent. Institutional review board approval of our hospital was obtained for this study (2015NZKY-030-01). If data were missing, an imputation method was used to integrate the data.
Surgical method
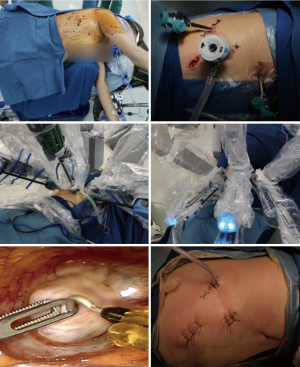
The individual surgical approach was determined by both tumor size and patient’s willings. Before surgeries, the patients were put into a feet-down tilt lateral decubitus position. In RATS, Three-arm technique is used in operation. If the PMNT located at the superior mediastinum, the camera trocar was first inserted at the fifth intercostal space (ICS), along the middle axillary line. Another trocar for dissecting arm was inserted at the seventh ICS, along the posterior axillary line. Then, the third trocar for grasping arm was inserted at the third ICS, along the anterior axillary line. Otherwise, the camera trocar was inserted at the sixth intercostal space (ICS), along the middle axillary line. The second trocar for dissecting arm was inserted at the eighth ICS, along the posterior axillary line and the third trocar for grasping arm was inserted at the fourth ICS, along the anterior axillary line. An assisted incision was applied alternatively according to the difficulty of operation and blood loss during RATS (Figure 1). The selection of incision which was designed as an equilateral triangle was according to the position of tumor (Figure 2). In VATS, the camera trocar was inserted at the sixth ICS, along the posterior axillary line. The second trocar was inserted at the seventh ICS, along the middle axillary line while the third trocar was inserted at the fourth ICS, along the anterior axillary line. Two surgeons who were familiar with both surgical techniques were involved in this study.

Statistical analyses
Continuous variables were expressed as the mean ± standard deviation. The Mann-Whitney U test or t-test was used to assess differences between the two groups. Categorical variables were compared using Pearson’s χ2 or Fisher’s exact tests. Results were considered statistically significant at a P<0.05. Data were analyzed using statistical program SPSS 21.0 software (SPSS, Chicago, IL, USA).
Results
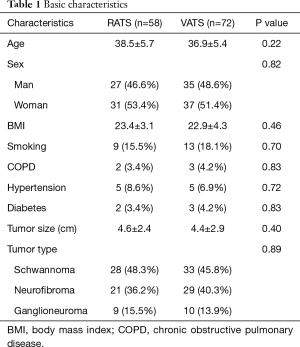
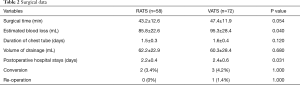
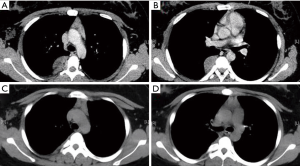
There were 58 patients combined with 27 men and 31 women (mean age 38.5±5.7 years) in RATS group, while VATS group included 72 patients with 35 men and 37 women (mean age 47.4±1.9 years). There were no significant differences in basic characteristics between two groups. The pathological type of posterior mediastinal neurogenic tumor in our study included schwannoma, neurofibroma and ganglioneuroma. The tumor size and pathological type between two groups had no significant difference (P=0.40 and P=0.89, respectively) (Table 1). For surgical data, the surgical time in RATS (43.2±12.6 min) was tended to be less than that in VATS (47.4±11.9 min) (P=0.054). Meanwhile, the estimated blood loss in RATS group (85.8±22.6 mL) was significantly less than that in VATS group (95.3±28.4 mL) (P=0.040). However, the duration of chest tube (days) and volume of drainage (mL) had no significant difference between two groups (P=0.12 and P=0.68, respectively). The postoperative hospital stays (days) of patients in RATS group (2.2±0.4 days) was significantly shorter than that in VATS group (2.4±0.6 days) (P=0.031). There were 7 patients whose tumor size was larger than 6 cm in RATS group and 5 in VATS group. The operation could successfully be accomplished by stretching the incision. However, the operative procedures of 2 patients in RATS group and 3 patients in VATS group were converted to open thoracotomy because of the hemorrhage. There was only one patient in VATS group who was re-operated at postoperative day 3 on account of postoperative hemorrhage (Table 2). The CT findings of a 28-year-old patient are presented in Figure 3. Complications, including pneumonia and surgical site infection, were recorded postoperatively. Postoperative complications occurred in 3 patients in RATS group and 7 in VATS group and there was no significant difference between two groups. Meanwhile, no significant difference was found between RATS group and VATS group in the incidence of the pneumonia and the surgical site infection (P=0.83 and P=0.26, respectively). Adverse reactions, consisting of blood pressure decrease, heart rate increase, chest pain and other reactions, were also collected. The adverse reactions occurred in 19 patients in RATS and 23 in VATS and no significant difference was found between two groups. There were no significant differences between two groups in terms of blood pressure decrease and heart rate increase (P=0.44 and P=0.44, respectively). Eight patients in RATS group and 7 in VATS group raise other adverse reactions and no significant difference was seen between two groups (Table 3).
Full table
Full table

Full table
Discussion
Posterior mediastinum neurogenic tumors are often schwannoma, neurofibroma and ganglioneuroma (3). Schwannoma is a rare tumor originating in the Schwann cells that surround peripheral nerve fibers (18-22). Less than 9% of schwannomas occur in the mediastinum, and this tumor is a relatively common mediastinal neurogenic tumor. Schwannomas are typically benign, slow growing, and well encapsulated. Neurofibroma is a benign neurogenic soft tissue tumor that may exist as a solitary tumor or as part of Neurofibromatosis (4,23,24). Ganglioneuromas, consisting of intermingled microscopic foci of neuroblastic elements in an expanding Schwannian stroma, are rare tumors that most often start in autonomic nerve cells and comprise more than 50% of the tumor volume (24-26). These three neurogenic tumors are usually noncancerous (benign). The video-assisted thoracoscopic surgery has been the most common method to resect posterior mediastinal neurogenic tumors recently. With the development of robot-assisted thoracoscopic surgery, several studies were reported to demonstrating the feasibility and safety of RATS. Ruurda et al. (27) reported the first case of robot-assisted thoracoscopic removal of a benign neurogenic tumor in the thorax. A 46-year-old woman presented with a history of paravertebral pain. A magnetic resonance imaging scan revealed a well-encapsulated mass that was suspected to be a neuroma at the level of T8–T9, separate from vascular structures, without extension in the foramina, and without a spinal canal component. A left robot-assisted thoracoscopic resection of the tumor was performed. Six trocars were placed during surgery, and then the tumor was carefully dissected and removed through one of the trocar openings. The histopathological findings revealed an ancient schwannoma. This case report demonstrates the feasibility and safety of robot-assisted thoracoscopic extirpation of a thoracic neurogenic tumor. A systemic review performed by Straughan et al. (28) manifesting robotic-assisted mediastinal surgery appears to be superior to open approaches of the mediastinum including PMGT and is comparable with video-thoracoscopic surgery when patient outcomes are considered. However, none original article was performed to explore the efficacy, feasibility and safety of RATS in PMGT. In this study, total 130 patients were enrolled and 58 patients underwent RATS. The individual surgical approach was determined by tumor size and patients’ willing. If the patient’s tumor size was less than 8 cm, the video-assisted or robot-assisted technique was used; otherwise, open operation was performed. The ultimate surgical approach was determined by patient’s willing when the tumor size was less than 8 cm. The results showed that the surgical time in RATS was tended to be less than in VATS while the estimated blood loss in RATS group was significantly less than that in VATS group. The potential reasons why RATS has superiority in operative blood loss over VATS could be explained as follows: first, VATS provides three-dimensional visualizations with ten-times-enlarged images and superior imaging quality, facilitating the identification of various structures during operations; second, RATS provides freely articulated movement of the robotic arms and seven degrees of freedom in dexterity with its EndoWrists, allowing surgeons to operate in a stable and comfortable environment, enabling more precise dissection and avoiding nerve and Adamkiewicz’s artery injury. Only three trocars were used during RATS, and an additional assisted incision was performed alternatively according to the situation of bleeding. There were 7 patients whose tumor sizes were larger than 6 cm in RATS group and 5 in VATS group. Nevertheless, the tumor sizes of these patients were all less than 8 cm thus the operation could successfully be accomplished by stretching the incision. In clinical practice, tumor size which is larger than 8 cm could be considered a potential contraindication to RATS or VATS for PMNT. The postoperative hospital stay was found to be shorter in RATS group than that in VATS group which may be due to the less blood loss and more satisfaction of patients in RATS group compared with VATS group. The operative procedures of 2 patients in RATS group and 3 patients in VATS group were converted to open thoracotomy because of the hemorrhage. There was no significant difference in postoperative complications and adverse reactions between two groups. Only one patient with a 7 cm schwannoma underwent reoperation at postoperative day 3 because of the bleeding after surgery. Therefore, robot-assisted thoracoscopic surgery has the superiorities in terms of surgical blood loss and postoperative hospital stay over video-assisted thoracoscopic surgery for posterior mediastinal neurogenic tumor. In conclusion, robot-assisted thoracoscopic surgery could be a feasible and safe way for resecting posterior mediastinal neurogenic tumor.
Limitation
There are some limitations in our study. First, this is a single-center retrospective study, which could have suffered from the retrospective nature of our study. Second, the small sample size in our study could result in selection bias.
Conclusions
This study demonstrates the feasibility and safety of robot-assisted thoracoscopic surgery for posterior mediastinal neurogenic tumor. The robot-assisted surgery may prove to be of additional value in challenging the video-assisted thoracoscopic surgery since it has the advantages in terms of manipulation and visualization over the video-assisted thoracoscopic surgery. Therefore, robot-assisted thoracoscopic surgery could be considered as a standard treatment for posterior mediastinal neurogenic tumor.
Acknowledgments
We thank all the members of the Department of Cardiothoracic Surgery in our hospital who participated in this research. We also thank Xiao-Kun Li for his advice on statistical analysis.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81172032) and the Natural Science Foundation of Jiangsu Province (BK20181239).
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-286
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-286). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Institutional Review Board of the jingling Hospital approved the study. The ID of ethics approval is (2015NZKY-030-01). The study outcomes will not affect the future patient management. This study is based on data retrieved from a hospital medical record system. All personal data have been protected and secured according to current national and international laws.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yamaguchi M, Yoshino I, Fukuyama S, et al. Surgical treatment of neurogenic tumors of the chest. Ann Thorac Cardiovasc Surg 2004;10:148-51. [PubMed]
- Gossot D, Izquierdo RR, Girard P, et al. Thoracoscopic resection of bulky intrathoracic benign lesions. Eur J Cardiothorac Surg 2007;32:848-51. [Crossref] [PubMed]
- Ikezoe J, Sone S, Higashihara T, et al. CT of intrathoracic neurogenic tumours. Eur J Radiol 1986;6:266-9. [PubMed]
- Liu S, Zhou X, Song A, et al. Giant plexiform neurofibroma of thigh in a young woman. Postgrad Med J 2019;95:459-60. [Crossref] [PubMed]
- Ratbi MB, El Oueriachi F, Arsalane A, et al. Surgery of benign neurogenic tumors in adults: single institution experience. Pan Afr Med J 2014;19:288. [Crossref] [PubMed]
- Bousamra M 2nd, Haasler GB, Patterson GA, et al. A comparative study of thoracoscopic vs open removal of benign neurogenic mediastinal tumors. Chest 1996;109:1461-5. [Crossref] [PubMed]
- Dickman CA, Apfelbaum RI. Thoracoscopic microsurgical excision of a thoracic schwannoma. Case report. J Neurosurg 1998;88:898-902. [Crossref] [PubMed]
- Yun PJ, Huang TW, Li YF, et al. Symptomatic pericardial schwannoma treated with video-assisted thoracic surgery: a case report. J Thorac Dis 2016;8:E349-52. [Crossref] [PubMed]
- Zierold D, Halow KD. Thoracoscopic resection as the preferred approach to posterior mediastinal neurogenic tumors. Surg Laparosc Endosc Percutan Tech 2000;10:222-5. [Crossref] [PubMed]
- Hazelrigg SR, Boley TM, Krasna MJ, et al. Thoracoscopic resection of posterior neurogenic tumors. Am Surg 1999;65:1129-33. [PubMed]
- Hu X, Wang M. Efficacy and Safety of Robot-assisted Thoracic Surgery (RATS) Compare with Video-assisted Thoracoscopic Surgery (VATS) for Lung Lobectomy in Patients with Non-small Cell Lung Cancer. Comb Chem High Throughput Screen 2019;22:169-78. [Crossref] [PubMed]
- Kim MP, Nguyen DT, Meisenbach LM, et al. Da Vinci Xi robot decreases the number of thoracotomy cases in pulmonary resection. J Thorac Dis 2019;11:145-53. [Crossref] [PubMed]
- Yang Y, Zhang X, Li B, et al. Robot-assisted esophagectomy (RAE) versus conventional minimally invasive esophagectomy (MIE) for resectable esophageal squamous cell carcinoma: protocol for a multicenter prospective randomized controlled trial (RAMIE trial, robot-assisted minimally invasive Esophagectomy). BMC Cancer 2019;19:608. [Crossref] [PubMed]
- Ujiie H, Gregor A, Yasufuku K. Minimally invasive surgical approaches for lung cancer. Expert Rev Respir Med 2019;13:571-8. [Crossref] [PubMed]
- Kanzaki M. Current status of robot-assisted thoracoscopic surgery for lung cancer. Surg Today 2019;49:795-802. [Crossref] [PubMed]
- Nelson DB, Mehran RJ, Mitchell KG, et al. Robotic-Assisted Lobectomy for Non-Small Cell Lung Cancer: A Comprehensive Institutional Experience. Ann Thorac Surg 2019;108:370-6. [Crossref] [PubMed]
- Shahin GMM, Brandon Bravo Bruinsma GJ, Stamenkovic S, et al. Training in robotic thoracic surgery-the European way. Ann Cardiothorac Surg 2019;8:202-9. [Crossref] [PubMed]
- Roger PA, Berna P, Merlusca G, et al. Schwannoma of the vagus nerve: diagnostic strategy and therapeutic approach. Rev Mal Respir 2012;29:70-3. [Crossref] [PubMed]
- Marouf R, Alloubi I. Benign primitive schwannoma of the pleura. Pan Afr Med J 2019;33:164. [PubMed]
- Santa Maria C, Santa Maria PL, Bulsara V, et al. Long-term quality of life in patients with vestibular schwannoma managed with microsurgery. J Laryngol Otol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Cilleruelo Ramos A, Borrego Pintado H, Castanedo Allende M. Endoscopic Management of Tracheal Neurofibroma. Arch Bronconeumol 2020;56:116. [PubMed]
- Pacchiarotti G, Wang MY, Kolcun JPG, et al. Robotic paravertebral schwannoma resection at extreme locations of the thoracic cavity. Neurosurg Focus 2017;42:E17. [Crossref] [PubMed]
- Temelkova I, Tchernev G. Giant Pelvic Neurofibroma in Patient with Plexiform Sciatic Neurofibroma and Neurofibromatosis Type 1. Open Access Maced J Med Sci 2019;7:1346-9. [Crossref] [PubMed]
- Rozmus J, Langer M, Murphy JJ, et al. Multiple persistent ganglioneuromas likely arising from the spontaneous maturation of metastatic neuroblastoma. J Pediatr Hematol Oncol 2012;34:151-3. [Crossref] [PubMed]
- Qing Y, Bin X, Jian W, et al. Adrenal ganglioneuromas: a 10-year experience in a Chinese population. Surgery 2010;147:854-60. [Crossref] [PubMed]
- Rondeau G, Nolet S, Latour M, et al. Clinical and biochemical features of seven adult adrenal ganglioneuromas. J Clin Endocrinol Metab 2010;95:3118-25. [Crossref] [PubMed]
- Ruurda JP, Hanlo PW, Hennipman A, et al. Robot-assisted thoracoscopic resection of a benign mediastinal neurogenic tumor: technical note. Neurosurgery 2003;52:462-4; discussion 464. [Crossref] [PubMed]
- Straughan DM, Fontaine JP, Toloza EM. Robotic-Assisted Videothoracoscopic Mediastinal Surgery. Cancer Control 2015;22:326-30. [Crossref] [PubMed]


