Postoperative atrial fibrillation prophylaxis using a novel amiodarone order set
Introduction
Postoperative atrial fibrillation (POAF) is a well-known complication following anatomic lung resection that occurs in up to 40% of patients without prophylaxis (1-3). In 2011 and 2014 respectively, the Society of Thoracic Surgeons (STS) and the American Association for Thoracic Surgery (AATS) published practice guidelines for the prevention of POAF following lung resection that recommend chemoprophylaxis such as beta blockers (BB), antiarrhythmics and calcium-channel blockers (CCB) (4,5). However, it is unclear if these recommendations are warranted after all types of lung resection. The STS provides an amiodarone dosing recommendation for lobectomy, but not sublobar resection. The AATS estimates the rate of POAF after wedge resection to be <5% and therefore only recommends prophylaxis for anatomic resection. Interestingly, they do not include reference to earlier studies that showed post-wedge resection rates of POAF to be over 20% (6,7). Contemporary data on the rate of POAF following wedge resection is lacking and the literature does not elucidate the need for prophylaxis.
Quantifying POAF after wedge resection and any subsequent need for prophylaxis is clinically relevant, as surgical indications continue to evolve. Historically, wedge resection was limited to the treatment of benign disease, metastasectomy, and other diagnostic testing (8-13). More recently it has been used in select patients as definitive treatment for early stage primary lung cancer (14-16). In this setting, a patient may undergo a lymph node dissection, which is a known risk factor for developing POAF (16-20). Despite this evolving paradigm, few studies have reported on the outcome of POAF following wedge resection alone or the need for prophylaxis (16,17,19,21). Older studies have reported a wide variability (4–25%) in the rate of POAF following wedge resection, and in the current minimally-invasive-era, the incidence and need for prophylaxis remains largely unknown (6,7,22,23).
In January 2015 an amiodarone-based POAF prophylaxis order set was introduced for anatomic resections at our institution. This was intended for post-anatomic lung resection patients who were at greatest risk for developing POAF: those ≥65 years of age (3,24-26). Wedge resection patients did not meet institutional criteria for automatic enrollment for this order set. The objectives of this study were to: (I) determine the rate of POAF following lung resection in our patient population with use of this amiodarone order set; (II) describe our institutional experience implementing this order set for anatomic resection patients, and (III) identify risk factors for developing POAF. We hypothesized that the rate of POAF in anatomic resection patients using our amiodarone order set would be comparable to previously published rates, and that the rate following wedge resection alone would be <5%.
We present the following article in accordance with STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-180).
Methods
Study design
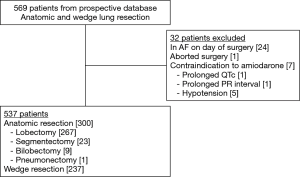
This was a retrospective cohort study of a prospectively maintained thoracic surgery database. All consecutive patients who underwent anatomic lung resection (segmentectomy, single or bi-lobectomy, or pneumonectomy) or non-anatomic wedge resection for any indication at our 400-bed, academic quaternary care hospital between 1 January 2015 and 20 April 2018 were eligible for review. Our primary outcome was POAF, defined as AF lasting ≥30 seconds on telemetry or if documented by 12-lead electrocardiogram (4). All patients were placed on routine telemetry monitoring for at least 72 hours postoperatively or until discharge. Post-discharge, we reviewed charts to identify any onset of POAF occurring within 30 days of surgery as determined by documentation from our electronic medical record, outside provider/hospital records, or confirmed by electrocardiogram. Patients are typically seen in the thoracic surgery clinic 2–3 weeks after discharge. Records are routinely reviewed >90 days postoperatively to assess for any 30- and 90-day morbidity and mortality. Patients were excluded if they had a history of chronic atrial fibrillation (AF) or if they had a history of paroxysmal AF and were in AF on the day of surgery. Patients were also excluded if surgery was aborted or if they had a contraindication to receiving amiodarone (hypotension and cardiac electrical conduction abnormalities) (Figure 1).
Patient demographics, history of paroxysmal/chronic AF, chronic obstructive pulmonary disease (COPD), myocardial infarction (MI), body mass index (BMI), pulmonary function tests (PFTs), preoperative medication use, procedural data including extent of lymph node sampling/dissection, chest tube (CT) duration, length of stay (LOS), morbidity, and mortality were evaluated. The degree of lymph node sampling/dissection was classified as either hilar (N1 lymph nodes up to the ipsilateral hilar lymph nodes) or mediastinal (N2 lymph nodes up to the ipsilateral mediastinal lymph nodes or more). Surgical approach was determined by attending surgeon and included video-assisted thoracoscopic surgery (VATS), robotic-assisted VATS, and thoracotomy. Pathology was designated as either benign, primary cancer, or metastatic. All patients were monitored on telemetry postoperatively. POAF with rapid ventricular response was managed according to institutional practice: medical management or cardioversion if hemodynamically unstable. Duration of surgery was calculated by the difference in time between intubation and extubation. All patients were extubated prior to leaving the operating room. Prolonged air leak was defined as one lasting greater than 5 days, consistent with STS quality metrics (27). Complications within 30 days as classified by Clavien-Dindo (CD) were evaluated (28,29).
Amiodarone order set
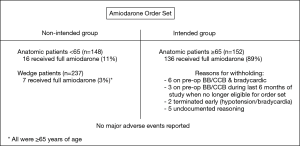
Prior to 2015, thoracic surgery coverage at our institution was inconsistent and lung resection patients did not receive POAF prophylaxis. In January 2015, the section of thoracic surgery was formed. A prophylactic amiodarone order set was implemented and intended for anatomic lung resection patients ≥65 years of age without a contraindication to taking amiodarone (e.g., hypotension or conduction abnormalities) (Figure 2). Patients on preoperative BBs or CCBs were initially eligible to receive amiodarone in addition to continuing their baseline medication. During the final 6 months of the study period, we elected to no longer administer the order set to these patients due to a potential risk of heart block. Patients were continued on their home medication regimen postoperatively. Electrolytes are monitored and repleted per standard protocol, but were not recorded for the purposes of this study. Use of amiodarone outside of intended parameters was at the discretion of the surgeon. The order set was adopted from an unpublished protocol used at Memorial Sloan Kettering Cancer Center and is a combination of the STS and AATS guideline recommendations for amiodarone dosing (4,5). The order set consisted of a postoperative intravenous (IV) bolus of 300 mg of amiodarone given over 1 hour in the post-anesthesia care unit (PACU). This was followed by 3 days of 400 mg amiodarone tablets taken orally 3 times per day. If a patient was discharged prior to completion of the regimen, they were given a prescription to complete as an outpatient. We elected to use this dosing and administration as it was the most practical for our institution. Many patients are initially unable to take pills in the PACU, policies at our hospital do not allow intravenous administration of amiodarone on the floor, and our average LOS is three days. Wedge resection patients were not intended to receive amiodarone prophylaxis and therefore only did so inadvertently or potentially if directed by the attending surgeon.
Statistical methods
Univariate analysis was performed to assess for differences in preoperative, intraoperative, and postoperative characteristics. Where applicable, missing data elements were censored and noted in table footnotes. Two-tailed Student’s t-tests were used for continuous variables and chi-square tests were used for categorical variables. To assess for risk factors of POAF and adjust for possible confounders, a multivariable logistic regression model was created using POAF as the dependent variable; independent variables were identified using an entry threshold of P<0.10 on univariate analysis or based upon presumed or known clinical relevance. The discriminatory power of the model was based on c-statistic values. The final model included age (≥65 vs. <65), surgical approach [thoracotomy vs. minimally invasive (VATS and robotic-assisted VATS)], operative time (continuous in 15-minute increments), and location of resection (categorical: upper, middle, or lower lobe). In addition, sensitivity analysis was performed by discretely adding variables that were felt to potentially impact the outcome but were not included in the model because they were not significant on unadjusted analysis: lymph node dissection (N1 and N2), CT duration, and preoperative BB use. A P value <0.05 was considered statistically significant. Statistical analysis was performed using STATA (Version 15.1, StataCorp).
The study was approved by the Institutional Review Board at Dartmouth College and Dartmouth-Hitchcock Medical Center (IRB# 31040) and was granted a waiver of consent given its retrospective nature.
Results
Perioperative patient characteristics stratified by resection type
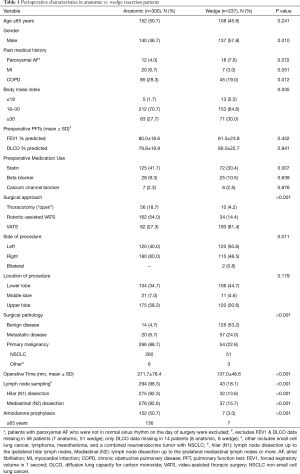
During the study period, 537 patients underwent lung resection with 300 (56%) undergoing anatomic resection and 237 (44%) wedge resection. Anatomic resection patients included segmentectomy (n=23), single lobectomy (n=267), bi-lobectomy (n=9), and pneumonectomy (n=1). Patients undergoing wedge resection were more likely to be male and less likely to have a history of COPD in comparison to those undergoing anatomic resection (Table 1). However, there were no differences in regard to the distribution of patients aged ≥65 years, a history of paroxysmal AF or MI, preoperative use of BB/CCB or baseline PFTs. Anatomic resection patients were significantly more likely to have undergone a robotic-assisted VATS or thoracotomy as compared to wedge patients. Anatomic resection patients were also more likely to have surgery in the right hemi-thorax and tissue pathology of primary malignancy. As expected, anatomic patients had a significantly longer operative time, occurrence of lymph node sampling (both overall and for hilar and mediastinal nodal stations), and administration of the amiodarone order set. Order set administration to the intended inclusion group occurred in 89% (136/152) of anatomic resection patients ≥65. No patients were taking amiodarone preoperatively. Of the 16 eligible patients who did not receive amiodarone, 3 were on BBs during the last 6 months of the study and thus ineligible. Five patients were on BBs prior to the last 6 months, but amiodarone was withheld secondary to bradycardia in the PACU. Additional details are displayed in Figure 3. In the wedge resection patients, 3.0% (7/237) received the order set outside of intended indications. All 7 were ≥65 years of age. There were no major adverse events associated with the use of amiodarone in our study.
Full table
POAF and non-POAF outcomes
All patients had at least 30-day follow-up. A summary of postoperative outcomes between anatomic and wedge resection patients is given in Table 2. In total, POAF occurred in 29 patients (5.4%) and only one had undergone a wedge resection. The rate of POAF was significantly lower in wedge resection patients as compared to anatomic patients (0.4% vs. 9.3%, P<0.001). The mean time to development of POAF was 2.3±2.4 days (range, 0 to 13 days). The single wedge resection patient who developed POAF was female, 81 years of age, underwent a VATS right upper lobe wedge resection without lymph node sampling, and did not receive the amiodarone order set. Her POAF resolved with administration of a BB 4 hours after onset.

Full table
The rate of POAF following anatomic resection in patients following implementation of the order set was significantly lower than our institutional historic rate (9.3% vs. 20.3%, P<0.001). The historic rate was calculated from consecutive anatomic resection patients between 2011 and 2014 (n=217). The distribution of patients ≥65 was similar during the two time periods (58.4% vs. 50.3%, P=0.067). However, patients in 2011 to 2014 were significantly more likely to have undergone a thoracotomy (76.2% vs. 18.7%, P<0.001). Further comparisons to the historical cohort were not able to be performed secondary to limited documentation, inconsistent thoracic surgery coverage, inconsistent telemetry use, and loss to follow-up.
As expected, other non-AF postoperative complications occurred significantly more in the anatomic resection patients as compared to wedge resections. Anatomic resection patients had a longer LOS and longer CT duration, but 30-day readmission and mortality were not different between groups.
POAF risk factors: univariate and multivariable analyses
On univariate analysis, patients who developed POAF were significantly older, more likely to have undergone a thoracotomy, and have an anatomic resection as compared to those who did not develop POAF (Table 3). Patients who developed POAF were also more likely to have undergone surgery for a primary malignancy, have a longer operative time, and lymph node dissection (both overall and for hilar (N1) and mediastinal (N2) nodal dissection). There were no differences in gender, preoperative comorbidities, PFTs, or location of resection between those who did and did not develop POAF.

Full table
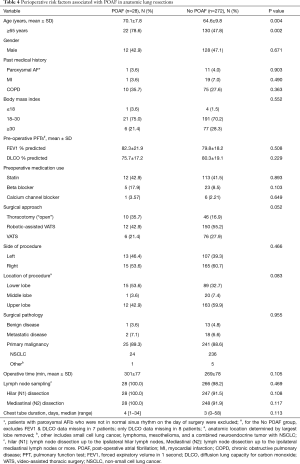
Given that only 1 patient in the wedge resection group developed POAF, a further statistically meaningful analysis including these patients was not possible. Consequently, we evaluated risk factors for POAF in the anatomic resection group (Table 4). In this group, patients who developed POAF were significantly older and had a higher percentage of age ≥65 years. There was a trend toward higher rates of thoracotomy, lower lobe resection, and longer operative time but these were not statistically significant.
Full table
On multivariable analysis, development of POAF was associated with age ≥65 (Table 5). In our patient population, surgical approach, longer operative time and lobe resected did not have an independent associated risk of POAF. This model yielded a c-statistic of 0.79.

Full table
Discussion
There is consistent evidence that chemoprophylaxis reduces the rate of POAF after anatomic lung resection (2,26,30-33). In this study, we observed that implementation of a new amiodarone order set following anatomic lung resection was easy to administer, well-tolerated, safe, and led to a reduction in the rate of POAF as compared to both institutional historic rates and previously published rates of POAF without prophylaxis (1-3). Moreover, our rate was comparable to other published studies that use more complex and prolonged amiodarone prophylaxis regimens (30-33). While wedge resection patients were not intended to receive amiodarone prophylaxis, limited data exist on the utility or need for prophylaxis following wedge resection alone. As the indications for a wedge resection continue to evolve (8-13), we must define the burden of POAF to avoid unnecessary medication exposure. POAF occurred in only a single wedge resection patient. Based on our data, we determined that our novel amiodarone order set is safe and efficacious for anatomic resections and that POAF prophylaxis following wedge resection is unnecessary.
Previous authors have demonstrated lower morbidity and mortality following wedge resection as compared to anatomic resection (16,17,19,21). However, the absence of discrete data on POAF following wedge resection alone, particularly in the contemporary minimally invasive era, limits the ability to ascertain the need for prophylaxis. The STS guidelines do not specify what type of lung resection warrants prophylaxis and there is a paucity of current data on the rate of POAF following wedge resections. Studies from more than ten years ago show a wide variability, ranging from 4% to 25% (6,7,22,23). While 7 wedge resection patients inadvertently received amiodarone prophylaxis in our study, it is unlikely this significantly alters our findings. Based on our results, we have re-educated all thoracic surgery providers at our institution that prophylaxis for wedge resection patients is not necessary. To inform national guidelines, data from other centers will be needed.
We observed a reduction in the rate of POAF after anatomic resection as compared to historic institutional rates when using our amiodarone order set. We acknowledge that this finding must be interpreted with caution. A historic analytic cohort could not be established due to intermittent thoracic coverage, unreliable use of telemetry and significant loss to follow up prior to 2015. There was a similar proportion of patients ≥65 in both groups and the historic cohort did not receive any POAF prophylaxis. However, there were significantly more open surgery cases in that group. Ultimately, the rate of POAF in the historical group was likely underestimated secondary to inconsistent telemetry use and lack of electronic record keeping. Unfortunately, this does limit the strength of our conclusions. Regardless, results from our study corroborate previous reports and our rate of POAF is in-line with other studies of amiodarone prophylaxis after anatomic resection (2,30-32).
To our knowledge, this is the first report of POAF outcomes using this specific amiodarone regimen, and there remains no national consensus on optimal administration of amiodarone for prophylaxis. The STS guidelines suggest dosing 1,050 mg by continuous infusion over the first 24 hours postoperatively followed by 400 mg tablets taken twice daily for 6 days. The AATS recommends a 150–300 mg IV bolus over 1 hour, followed by a 10–50 mg/hour IV continuous infusion over 24 hours (4,5). We elected to use a practical combination of both recommendations: a 300mg IV loading bolus over 1 hour in the PACU followed by a 400 mg tablets three times daily for 3 days. This regimen allowed for excellent compliance as patients were initially recovering from surgery and the early transition to tablets had minimal side effects. Limiting the IV infusion is also less problematic for nursing, complies with hospital policies for floor level admissions, decreases the risk of peripheral infusion-related complications such as thrombophlebitis (34,35), and allows for expedited discharge. We did not have any major adverse events associated with the use of amiodarone. All of our patients were able to tolerate amiodarone orally, but if that is not possible, other studies have reported alternative administration via nasogastric tube (31).
During the study period, there was a transition in order set inclusion for patients on preoperative BB/CCBs. The STS and AATS POAF prevention guidelines recommend continuing a preoperative BB, but it is unclear if that should also exclude patients from receiving additional prophylaxis. In our study, a small number of anatomic resection patients were on a preoperative BB/CCB and none were on amiodarone. Initially, these patients continued their medications while simultaneously receiving the amiodarone order set. However, 6 months prior to the end of the study period a patient experienced asymptomatic transient heart block. Following this event patients on preoperative BBs (n=3) or CCBs (n=1) were continued on these medications and not given amiodarone. None of these 4 patients experienced POAF. We are unable to determine if preoperative BB/CCB use significantly affected our results, though these medications were used in only ~13% of patients in both the wedge and anatomic lung resection groups. Further study is needed in a larger cohort to help clarify this issue.
Some argue against the use of amiodarone after anatomic lung resection, citing issues of clinical relevance and safety (14,36). Two randomized controlled trials (RCTs) comparing no prophylaxis to using amiodarone did not report an increased incidence of POAF at discharge between groups (30,31). However, these and other retrospective studies have been confounded by under-reporting of POAF, excluding cases that occur after another pulmonary complication or not using telemetry monitoring (14,32). Inclusion of asymptomatic POAF and using conservative definitions of POAF is clinically important, as authors have demonstrated that POAF following non-cardiac surgery is associated with an increased long-term risk of ischemic stroke (37). In our study, POAF was clinically relevant, as several patients who developed POAF experienced hemodynamic instability that required intensive care unit transfer and two patients underwent cardioversion. At follow up, 2 patients remained in AF. With regard to safety, the two most recent RCTs utilizing amiodarone prophylaxis in POAF after anatomic resection showed a sizable reduction in POAF (<15%) with no major adverse events associated with amiodarone (30,31). Our rate of POAF following anatomic resection was less than 10% and we had no major adverse events associated with the use of amiodarone. POAF prevention is effective, clinically relevant and safe.
The occurrence of POAF after wedge resection was too rare to allow for inclusion of resection type in multivariable analysis. This will be an area for future study, as wedge resection surgery is becoming more popular and stratifying POAF risk will be needed. As segmentectomy becomes more commonly performed, it will be interesting to see if this also affects POAF compared with lobectomy. For anatomic resections, we modeled perioperative risk factors for developing POAF. Analysis revealed that age ≥65 was the only independent predictor in these patients. This is in-line with previous findings (3,24-26). Authors have theorized that POAF following lung resection occurs secondary to inflammation, and this may be amplified during longer surgery (25,38,39). On univariate analysis, anatomic resection patients had a significantly longer duration of surgery, which may have led to more inflammation and thus a higher rate of POAF. However, increased operative time was not associated with POAF in our cohort of anatomic resections. Mediastinal lymph node dissection has been associated with POAF as well (15,16). While many wedge resection patients underwent a mediastinal lymph node dissection in our study, none of these patients experienced POAF and it was not an independent risk factor in our anatomic resection patients. Interestingly, surgical approach via open thoracotomy was also not independently associated with a higher risk of POAF on multivariable analysis.
The findings of our study should be considered in the context of several limitations. First, this is a single institution, retrospective review and our results may not be generalizable to a broader patient population. POAF occurred in only one patient who did not undergo lymph node dissection, which limits the ability to independently assess this as a risk factor. In addition, selection bias on who received amiodarone could have influenced our results. Approximately 11% of anatomic resection patients ≥65 did not receive amiodarone and a similar percentage <65 did receive it. Also, 3% of wedge resection patients received amiodarone, which may have prevented POAF in that group. It is unclear if this was from incorrect ordering or potentially surgeon bias as all 7 were ≥65 years of age. Nevertheless, if all of these patients would have developed POAF, the rate in the wedge cohort would have been only 3.4%, which in our opinion still does not warrant prophylaxis in this group. The incidence of POAF in wedge resections may also have been confounded by a decreased detection rate, as they had a shorter postoperative LOS. Lastly, we were unable to establish a pre-order set analytic cohort for direct comparison in the anatomic resection patients. This limited our ability to draw any definitive conclusions on the reduced rate of POAF seen in our anatomic resection patients that received the amiodarone order set. Despite these limitations, our study addresses a knowledge gap in the incidence of POAF following wedge resection alone and highlights the safety and efficacy of a novel amiodarone order set that is easy to administer.
Conclusions
This study demonstrates the feasibility and efficacy in employing a well-tolerated and practical POAF prophylactic amiodarone order set in anatomic lung resection patients. The occurrence of POAF following wedge resection is too rare to warrant prophylaxis. Results from this study can be used to inform national guidelines for further refinement for POAF prevention.
Acknowledgments
The authors wish to thank Niveditta Ramkumar MPH of The Dartmouth Institute for Health Policy and Clinical Practice for review and assistance with the statistical analysis. JDP is supported by The Dartmouth-Hitchcock Cancer Research Fellows Program and by the NCI Cancer Center Support Grant 5P30CA023108 to the Dartmouth-Hitchcock Norris Cotton Cancer Center as well as The Dartmouth Clinical and Translational Science Institute, under award number UL1TR001086 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-180
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-180). JDP reports personal fees from Intuitive Surgical, Inc., outside the submitted work, but has no ongoing relationship. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board at Dartmouth College and Dartmouth-Hitchcock Medical Center (IRB# 31040) and was granted a waiver of consent given its retrospective nature.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sugarbaker DJ, Jaklitsch MT, Bueno R, et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg 2004;128:138-46. [Crossref] [PubMed]
- Lanza LA, Visbal AI, DeValeria PA, et al. Low-dose oral amiodarone prophylaxis reduces atrial fibrillation after pulmonary resection. Ann Thorac Surg 2003;75:223-30; discussion 230. [Crossref] [PubMed]
- Onaitis M, D’Amico T, Zhao Y, et al. Risk factors for atrial fibrillation after lung cancer surgery: analysis of the Society of Thoracic Surgeons general thoracic surgery database. Ann Thorac Surg 2010;90:368-74. [Crossref] [PubMed]
- Frendl G, Sodickson AC, Chung MK, et al. 2014 AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. J Thorac Cardiovasc Surg 2014;148:e153-93. [Crossref] [PubMed]
- Fernando HC, Jaklitsch MT, Walsh GL, et al. The Society of Thoracic Surgeons practice guideline on the prophylaxis and management of atrial fibrillation associated with general thoracic surgery: executive summary. Ann Thorac Surg 2011;92:1144-52. [Crossref] [PubMed]
- Curtis JJ, Parker BM, McKenney CA, et al. Incidence and predictors of supraventricular dysrhythmias after pulmonary resection. Ann Thorac Surg 1998;66:1766-71. [Crossref] [PubMed]
- Mahesh B, Forrester-Wood C, Amer K, et al. Value of Wedge Resection for Lung Cancer in Poor Cardiopulmonary Status Patients. Asian Cardiovasc Thorac Ann 2006;14:123-7. [Crossref] [PubMed]
- Santillan-Doherty P, Cuellar-Rodríguez J, Argote-Greene LM, et al. Nonanatomic Thoracoscopic Wedge Resection for Diffuse Lung Disease and Indeterminate Pulmonary Nodule. World J Surg 2002;26:43-8. [Crossref] [PubMed]
- Ito H, Nakayama H. Surgical management of pulmonary metastases. Gan To Kagaku Ryoho 2010;37:200-3. [PubMed]
- Higashiyama M, Tokunaga T, Nakagiri T, et al. Pulmonary metastasectomy: outcomes and issues according to the type of surgical resection. Gen Thorac Cardiovasc Surg 2015;63:320-30. [Crossref] [PubMed]
- Kondo H, Okumura T, Ohde Y, et al. Surgical treatment for metastatic malignancies. Pulmonary metastasis: indications and outcomes. Int J Clin Oncol 2005;10:81-5. [Crossref] [PubMed]
- Kuzucu A, Ulutas H, Reha Celik M, et al. Hydatid cysts of the lung: lesion size in relation to clinical presentation and therapeutic approach. Surg Today 2014;44:131-6. [Crossref] [PubMed]
- Bolaños-Morales FV, Gómez-Portugal EP, Aguilar-Mena ME, et al. Lung necrosectomy in pediatric patients with necrotizing pneumonia. Gen Thorac Cardiovasc Surg 2018;66:155-60. [Crossref] [PubMed]
- Garner M, Routledge T, King JE, et al. New-onset atrial fibrillation after anatomic lung resection: Predictive factors, treatment and follow-up in a UK thoracic centre. Interact Cardiovasc Thorac Surg 2017;24:260-4. [PubMed]
- Muranishi Y, Sonobe M, Menju T, et al. Atrial fibrillation after lung cancer surgery: incidence, severity, and risk factors. Surg Today 2017;47:252-8. [Crossref] [PubMed]
- Altorki NK, Kamel MK, Narula N, et al. Anatomical Segmentectomy and Wedge Resections Are Associated with Comparable Outcomes for Patients with Small cT1N0 Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:1984-92. [Crossref] [PubMed]
- Hou B, Deng X-F, Zhou D, et al. Segmentectomy versus wedge resection for the treatment of high-risk operable patients with stage I non-small cell lung cancer: a meta-analysis. Ther Adv Respir Dis 2016;10:435-43. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Appropriate Sublobar Resection Choice for Ground Glass Opacity-Dominant Clinical Stage IA Lung Adenocarcinoma. Chest 2014;145:66-71. [Crossref] [PubMed]
- Linden PA, D'Amico TA, Perry Y, et al. Quantifying the Safety Benefits of Wedge Resection: a Society of Thoracic Surgery Database Propensity-Matched Analysis. Ann Thorac Surg 2014;98:1705-11; discussion 1711-2.
- Onaitis MW, Furnary AP, Kosinski AS, et al. Prediction of Long-Term Survival After Lung Cancer Surgery for Elderly Patients in The Society of Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg 2018;105:309-16. [Crossref] [PubMed]
- Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915-24. [Crossref] [PubMed]
- Vaporciyan AA, Correa AM, Rice DC, et al. Risk factors associated with atrial fibrillation after noncardiac thoracic surgery: analysis of 2588 patients. J Thorac Cardiovasc Surg 2004;127:779-86. [Crossref] [PubMed]
- McKenna RJ, Mahtabifard A, Yap J, et al. Wedge Resection and Brachytherapy for Lung Cancer in Patients With Poor Pulmonary Function. Ann Thorac Surg 2008;85:S733-6. [Crossref] [PubMed]
- Amar D. Perioperative atrial tachyarrhythmias. Anesthesiology 2002;97:1618-23. [Crossref] [PubMed]
- Amar D. Editorial: Thoracic: Perioperative Management -Postoperative atrial fibrillation: Is there a need for prevention? J Thorac Cardiovasc Surg 2016;151:913-5. [Crossref] [PubMed]
- Amar D. Prevention and Management of Perioperative Arrhythmias in the Thoracic Surgical Population. Anesthesiol Clin 2008;26:325-35. [Crossref] [PubMed]
- The Society of Thoracic Surgeons. General Thoracic Surgery Database Training Manual STS National Database. 2018:45-57. Available online: https://www.sts.org/sites/default/files/content/GTSDTrainingManual_September24_2018.pdf
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Riber LP, Christensen TD, Jensen HK, et al. Amiodarone Significantly Decreases Atrial Fibrillation in Patients Undergoing Surgery for Lung Cancer. Ann Thorac Surg 2012;94:339-44; discussion 345-6. [Crossref] [PubMed]
- Tisdale JE, Wroblewski HA, Wall DS, et al. A Randomized Trial Evaluating Amiodarone for Prevention of Atrial Fibrillation After Pulmonary Resection. Ann Thorac Surg 2009;88:886-93; discussion 894-5. [Crossref] [PubMed]
- Berry MF, D’Amico TA, Onaitis MW. Use of Amiodarone After Major Lung Resection. Ann Thorac Surg 2014;98:1199-206. [Crossref] [PubMed]
- Riber LP, Larsen TB, Christensen TD. Postoperative Atrial Fibrillation Prophylaxis After Lung Surgery: Systematic Review and Meta-Analysis. Ann Thorac Surg 2014;98:1989-97. [Crossref] [PubMed]
- Veloso HH, De Paola AAV. Thrombophlebitis: a common complication of amiodarone. Am Fam Physician 2004;70:1448. [PubMed]
- Showkathali R, Earley MJ, Sporton S. Images in Emergency Medicine: Amiodarone induced thrombophlebitis. Emerg Med J 2006;23:660. [Crossref] [PubMed]
- Hasson Charles R, Shabsigh M, Sacchet-Cardozo F, et al. Con: Atrial Fibrillation Prophylaxis Is Not Necessary in Patients Undergoing Major Thoracic Surgery. J Cardiothorac Vasc Anesth 2017;31:751-4. [Crossref] [PubMed]
- Gialdini G, Nearing K, Bhave PD, et al. Perioperative Atrial Fibrillation and the Long-term Risk of Ischemic Stroke. JAMA 2014;312:616. [Crossref] [PubMed]
- Anselmi A, Possati G, Gaudino M. Postoperative Inflammatory Reaction and Atrial Fibrillation: Simple Correlation or Causation? Ann Thorac Surg 2009;88:326-33. [Crossref] [PubMed]
- Amar D, Zhang H, Shi W, et al. Brain natriuretic peptide and risk of atrial fibrillation after thoracic surgery. J Thorac Cardiovasc Surg 2012;144:1249-53. [Crossref] [PubMed]