
Thoracoscopic segmentectomy with simple routine bronchoscopic inflation for intersegmental plane identification: short and mid-term outcomes compared with lobectomy
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide (1). For cure in the early stage of the disease, the best chance is offered by surgery (2). Lobectomy is recommended as the standard approach (3,4).
Segmentectomy is currently indicated in predominantly ground glass opacities and in solid tumors in high-risk patients (1,3). Apace with population aging and lung cancer screening programs, the number of such patients is increasing. Other potential indications are neuroendocrine, special cases such as bilateral or multifocal tumors or deeply located metastases. Although many propensity-matched evaluations suggest that anatomical segmentectomy is equivalent to lobectomy in solid tumors of a size less than 2 cm diameter, we are still waiting the results of prospective randomized studies (5,6).
The long-term benefits of video-assisted thoracoscopic surgery (VATS) segmentectomy over lobectomy in patients with poor lung function have not been firmly established (7). This may be due to the difference in the number of resected segments or due to those potential issues during VATS in venous drainage, arterial supply or intersegmental plane identification. For target segment identification several techniques including various inflation-deflation approaches or intravenous or intrabronchial indocyanine green injections have been developed (8,9). None of these have gained wide acceptance and there is room for a simple and reproduceable technique.
We describe a simple bronchoscopic inflation technique for target segment identification during VATS segmentectomy. Our aim was to compare the short- and mid-term outcomes between VATS segmentectomy and lobectomy after implementation of this new technique. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-656).
Methods
Design
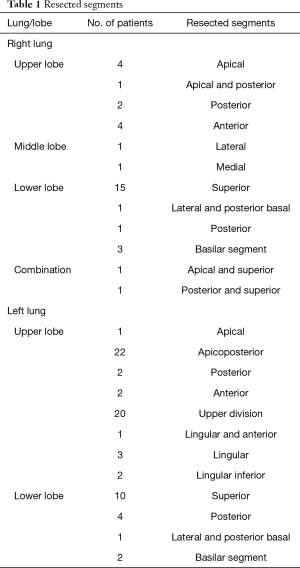
All patients with primary lung cancer treated with VATS in Central Finland Central Hospital from September 1, 2012 to June 31, 2019 were included in the study. A further 29 consecutive VATS segmentectomies performed in Helsinki University Hospital by the same surgeon (ES) between November 2007 and May 2012 were included. The final study population consisted of patients with intention to treat with either VATS segmentectomy (n=105) or VATS lobectomy (n=110). The indication for VATS segmentectomy was an increased surgical risk in stage I non-small cell lung cancer (NSCLC) in 56 patients, carcinoid tumors in 15, a solid type NSCLC of a size smaller that 2 cm in 13 patients, a ground-glass opacity with maximum of 6 mm solid component in 11 patients and a special indication in 10 patients (bilateral tumors in 3, synchronous ipsilateral tumors in 2, synchronous other major surgery in 3, metachronous tumor in one and fissure-crossing tumor in one). Resected segments and number of patients are listed in Table 1. The two approaches were compared regarding baseline differences in patient risk profile, short-term outcomes including postoperative complications, length of hospital stay, and mid-term outcomes regarding recurrence-free and overall survival. Pre- and postoperative lung function was measured. A prospective surgical database was maintained throughout the study period confirmed from the hospital records, including information on cancer recurrence. Survival data was further confirmed from Statistics Finland. Median follow-up time in segmentectomy group was 27.1 (IQR, 13.9–55.8), in lobectomy group 27.1 (IQR, 15.9–46.5), and in respective groups including only stage I NSCLC (excluding neuroendocrine tumors) 33.2 (IQR, 14.4–54.5) and 33.8 (IQR, 16.7–54.4) months. In patients with neuroendocrine tumors, follow-up time was 20.5 (IQR, 10.0–79.0) months. The study was approved by the local hospital districts.
Full table
Surgical technique
This was an intent to treat analysis. Of 105 patients, the planned segmentectomy was converted in five patients: to VATS lobectomy due to inadequate marginals in one, to VATS wedge resection due to unstable hemodynamics in one, to open wedge resection due to dense adhesions in one, and to two open segmentectomies due to dense adhesions in one and bleeding in one patient.
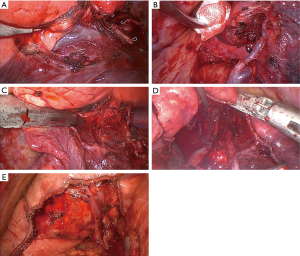
Surgical planning was based on multidetector CT. Tumor location and the bronchial and vessel anatomy of the target segment were identified in CT. Bronchoscopic evaluation by the operating surgeon at the beginning of surgery confirmed the bronchial anatomy. One surgeon (ES) performed all operations using the posterior four-port approach using 2-D thoracoscopy up to May 2018 and 3-D optics (Aesculap, B.Braun, Melsungen, Germany) from there on. Segmental vessel branches and bronchus were isolated separately. The order of division of these structures depended on the target segment. Vessels were divided using either staplers, clips or Ligasure (Medtronic, Fridley, MN) as appropriate. The segmental bronchus was encircled by a vessel loop and bronchoscope Olympus, Tokyo, Japan) was brought to the isolated segmental bronchus and often further to a branch adjacent to the intersegmental plane. Next, O2 inflow with 2 L/min controlled by a rotameter was connected to the suction valve of the bronchoscope. In this set-up, this valve controlled the inflow of oxygen. The controlled inflow of oxygen inflated the segment or at least the area of lung tissue adjacent to the intersegmental plane. After inflation the segmental bronchus was divided using a stapler. Lifting the distal bronchial stump enables central dissection along the intersegmental vein branches (Figure 1A,B,C,D,E). These centrally dissected intersegmental vein(s) and the inflation-deflation line guided the division of more peripheral parts of intersegmental border by stapler. Routine lymphadenectomy or at least sampling of segmental, lobar, hilar, and mediastinal lymph nodes was also carried during surgery. The resected segment(s) was removed from the chest cavity inside a specimen bag and palpated to confirm a minimum macroscopic margin of 2 cm at the collapsed lung tissue. No mesh or sealants were used to cover the intersegmental plane. At the end of surgery, a single chest tube was inserted and the lung was inflated under direct thoracoscopic vision to reveal the likelihood of any kinking of the remaining segments. During the closing of the port site, the anesthetist reported the extent of respiratory air leak. A leak of less than 100 mL per a single breath was accepted and the chest tube was connected to a suction of −10 cmH2O.
Definitions
The 8th edition of TNM classification was used in staging. The Charlson comorbidity index and ASA-grade were used in risk assessment. High-risk patients were defined with at least one of the following: age ≥80 years, FEV1 ≤50%, DLCO ≤50%, Charlson comorbidity index ≥5, maximal VO2 10–12 mL/kg/min, or stair-climbing of only two flights of stairs (7.2 m). In the stair-climbing test, the measured maximum climb was four flights (14.4 m). The categorization of segmentectomy into technically simple or complex procedure was done according to a recent Japanese randomized study (6). In addition, the upper division of left upper lobe and basal segmentectomy was categorized as simple segmentectomy.
Statistical analysis
Kaplan-Meier survival curves were calculated according to the life table method to visualize the crude recurrence-free and overall survival up to 3 years after surgery. Multivariable Cox regression was used to calculate hazard ratios (HRs) with 95% confidence intervals (CIs) of recurrence and overall mortality. Lobectomy was used as the reference group. The regression models were adjusted for eight potential confounding factors: age (continuous), sex (male, female), Charlson comorbidity index (0–4, ≥5), FEV1 (0–50, >50), DLCO (0–50, >50), histological type (neuroendocrine, other), pathological stage (stage I, >stage I), and adjuvant treatment (yes/no). For patients receiving neoadjuvant treatment, clinical Stage was used instead of pathological Stage in the regression analysis. Comparison of proportions, mean, and median values of other measured variables was made with chi-square test, Mann-Whitney U-test and T-test as appropriate. All statistical analyses were conducted using IBM SPSS 25.0 (IBM corp., Armonk, NY, USA).
Results
Preoperative patient evaluation and risk profile
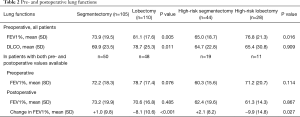
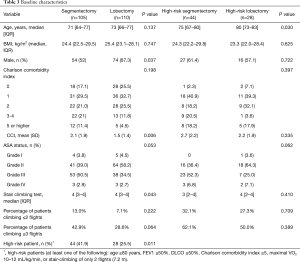
A total of 215 patients with intention to treat with either VATS segmentectomy (n=105) or VATS lobectomy (n=110) were included in the study. All patients underwent preoperative pulmonary function tests (Table 2) and physical evaluation including stair-climbing test (Table 3). Patients in the segmentectomy group had lower preoperative mean FEV1% and DLCO than the lobectomy group (Table 2). Patients in the segmentectomy group had more comorbidities, higher ASA grade, and performed worse on the stair climbing test (Table 3). In all, 41.9% of patients in the segmentectomy group were considered high-risk patients compared to 25.5% in the lobectomy group (P=0.011, Table 3). High-risk patients are also presented separately in the tables.
Full table
Full table
Staging
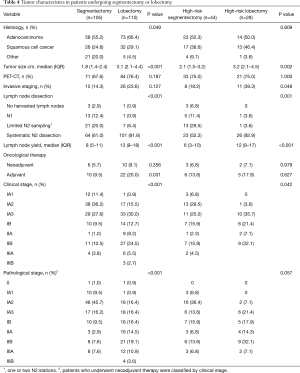
PET-CT was performed on 67.6% of patients in the segmentectomy and 76.4% in the lobectomy group (P=0.187), and invasive mediastinal staging in 14.3% and 23.6% respectively (P=0.127). Patients in the lobectomy group had generally larger tumors (median 1.8 vs. 3.1 cm, P<0.001) and also more advanced clinical stage (Table 4). There was no difference in the proportion of patients receiving neoadjuvant treatment (5.7% vs. 9.1%, P=0.356).
Full table
Segmentectomy indications
Of 105 segmentectomies, 56 (53%) underwent sublobar resection due to high age, comorbidities, poor exercise capacity or limited cardiopulmonary reserve (Tables 2,3). Fifteen patients (14.3%) had carcinoid tumors and 11 (10.5%) predominant ground glass opacities. Of 13 (12.4%) solid low-risk solid cancer patients, 12 had a tumor size smaller than 2 cm and one of a size of 3 cm at the optimal location. Ten patients were considered to have a special indication: four synchronous cancers, one metachronous cancer, two synchronous other solid cancers requiring surgery, one fissure-crossing tumor, one patient less than 2 months post contralateral thoracotomy due to empyema and bronchopleural fistula, and one was suspected of having a metastasis.
Outcomes
This was an intention-to-treat analysis. Lymph node yield in segmentectomy was lower than in the lobectomy group [median yield 8 (IQR: 5–11) vs. 13 (IQR: 9–18), P<0.001]. This was also observed when including high-risk patients only (Table 4). With a learning curve, the difference in lymph node yield decreased (Figure 2). Furthermore, the complexity of segmentectomy from the first half of the series to second half rose from 18.9% to 34.6% (P=0.010).
Pathological staging
In the segmentectomy group, final tumor histology was more often neuroendocrine tumor (14.3% vs. 2.7%, P=0.002). The lobectomy group had generally more advanced pathological stage (Table 4).
Short-term outcomes
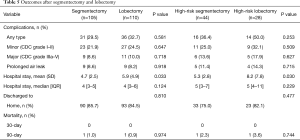
Overall (29.5% vs. 32.7%, P=0.581) and major complication rates (8.6% vs. 10.0%, P=0.718) were similar between the segmentectomy and lobectomy groups. This was also observed in high-risk patients only (Table 5). No patients died during the first 30 postoperative days, and one high-risk patient in each group died within 90 days. Median hospital stay was 4 days in both groups (P=0.108). Mean stay was shorter in the segmentectomy group (4.7 vs. 5.9 days, P=0.029). The majority of patients were discharged home in both groups (86% vs. 84%, P=0.659), Table 5.
Full table
Mid-term outcomes
Overall 3-year survival in the segmentectomy and lobectomy groups was 86.0% vs. 71.7% (P=0.042, Figure 3A) and recurrence-free 3-year survival 92.4% vs. 76.3% (P=0.002, Figure 3B). All patients were alive after surgery for neuroendocrine tumors. When including only stage I NCSLC (excluding neuroendocrine tumors), 3-year overall survival rates were 86.8% vs. 79.8% (P=0.412, Figure 3C) and 3-year recurrence-free survival 93.0% vs. 89.7% (P=0.450, Figure 3D). In high-risk patients, the respective overall survival rates were 69.1% and 62.9% (P=0.645) and recurrence-free survival 86.7% vs. 71.8% (P=0.132).
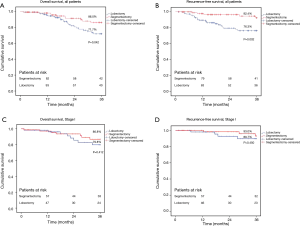
In adjusted analysis, HR for overall mortality was similar between segmentectomy and lobectomy (HR 0.89, 95% CI: 0.43–1.84). Recurrence risk in adjusted analysis was lower after segmentectomy (HR 0.26, 95% CI: 0.09–0.80, Table 6). Of 7 recurrences after segmentectomy, 3 were systemic, 2 mediastinal and one in separate ipsilateral lobe. One patient had synchronous recurrence in liver, mediastinum and in the resected lobe.

Full table
Postoperative pulmonary function
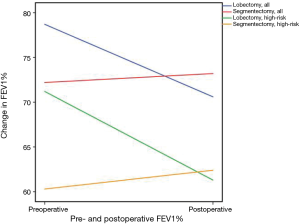
Following segmentectomy, FEV1% improved by 1.0%, whereas after lobectomy FEV1% declined by a mean of 8.1% (P<0.001). In high-risk patients, respective changes in FEV1% were 2.1% improvement and 9.9% decline (P=0.027, Figure 4, Table 2).
Discussion
This study with a simple intersegmental plane identification technique shows shorter hospital stay in patients with more comorbidities, decreased preoperative pulmonary functions, and worse exercise capacity after VATS segmentectomy compared to better surgical candidates after VATS lobectomy. Furthermore, the preservation of lung function also in high-risk patients, was observed without any compromise in mid-term oncologic outcomes.
In comparison of short- and mid-term results after segmentectomy and lobectomy, varying surgical techniques and indications, differences in lymph node dissection and tumor margins cause problems even after matching. In this study, the main strengths were consistent indications and surgical technique as all patients in both groups were operated on by a single surgeon, and the results favored segmentectomy. Further strengths were prospective database with the possibility to confirm data from hospital records and complete follow-up information without any limitations of register-based data. With the limited sample size and multiple causes for increased surgical risk, the potential long-term benefits of segmentectomy in high-risk patients remain inconclusive. Thus, the main finding of this study concerns the short-term benefits with preservation of lung function without compromising oncologic outcome. Overall, the technique described still needs to be replicated in future studies.
Lobectomy has remained the gold standard for operable NSCLC in terms of long-term outcomes (3,4). Many single-center and register-based studies have recently reported segmentectomy as an alternative to lobectomy in solid tumors less than 2 cm in size with similar survival (10-14). Our similar mid-term results likewise support the use of segmentectomy for T1a and T1b solid tumors. In guidelines, segmentectomy is still mainly considered appropriate for pure or predominantly ground glass opacities and for solid tumors only in high-risk patients (1,3). In the high-risk patients in our study segmentectomy shortened hospital stay and preserved lung function, thereby supporting the use of this technique. It seems plausible that the preservation of lung function also has beneficial long-term effects. Another major group undergoing segmentectomy in our study was carcinoid tumors. A recent study reported anatomical resection (lobectomy or segmentectomy) to be superior to wedge resection in Stage 1 neuroendocrine tumors (15,16). In our study, none of the patients operated on for neuroendocrine tumors died during 3-year follow-up after surgery, suggesting the possibility of routine use of segmentectomy in any technically suitable anatomical resection.
For good short-term and long-term outcomes and for the preservation of functional lung tissue, the important concepts are the preservation of venous drainage and arterial supply to the remaining segments, proper lymph node assessment and accurate determination of intersegmental planes. For this identification a number of techniques have been proposed, including intravenous or intrabronchial indocyanine green injections and inflation-deflation, but none has gained wide acceptance (8,9). This inflation technique, in principle, is very similar to bronchoscopic jet inflation with no need for special equipment. A rotameter is standard equipment in every operating room. The suction valve helps to control the inflation itself. In case of any over-inflation in an emphysematous lung, after closure of the target bronchus the open bronchial branches of the remaining segments deflate the intersegmental area. The inflation-deflation line and the central dissection along intersegmental veins provide landmarks for more peripheral stapling of intersegmental borders. The more central intersegmental raw surface does not seem to need any coverage with similar air-leak rates compared to lobectomy and well-preserved lung functions even in high-risk patients. With this simple technique in this study, more widespread use of VATS segmentectomies could be achieved. In fact, the rate of segmentectomy in a population-based setting has recently been as low as 3% (17) and in the STS and ESTS databases respectively 3.9% and 7.4% (18). Some have raised the issue of inadequate lymph node yield in VATS segmentectomy compared to lobectomy (19). Systematic lymph node dissection is also considered a standard approach in stage I NSCLC (3). In our study lymph node yield was lower after segmentectomy than after lobectomy, but is at least partly explained by the learning curve.
Although in several studies segmentectomy is preferable to lobectomy, the functional benefit of VATS segmentectomy over VATS lobectomy in patients with poor lung function has been questioned (7,20,21). In a recent review, a mean early loss of FEV1% within two months after lobectomy and segmentectomy were 25% and 18%, and after 12 months 11% and 5% respectively (7). In our study, after a medium of nine months postoperatively, changes in FEV1% after VATS segmentectomy and VATS lobectomy were +1% and −8.1%. In high-risk patients, the difference between segmentectomy and lobectomy was even higher. This can be considered a clear clinically significant advantage supporting the use of segmentectomy over lobectomy when appropriate, especially in high-risk patients. Because in real-world practice the causes of increased surgical risks are multi-factorial, as in this study, it will be difficult to evaluate the possibility to lower the functional limit of surgery with properly conducted segmentectomy.
In conclusion, in this study we present a simple bronchoscopic inflation technique for intersegmental plane identification. With this technique, an improvement in short-term outcomes compared to VATS lobectomy can be achieved with preservation of pulmonary function even in high-risk patients without compromising the oncological outcome. VATS segmentectomy is still rarely used over VATS lobectomy despite clear indications and advantages. With this new simple and reproducible technique, we aim at wider use of anatomical sublobar resections.
Acknowledgments
Funding: Finnish State Research Funding, Instrumentarium Science Foundation, Georg C. and Mary Ehrnrooth Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-656
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-656). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Hospital districts. Because of the retrospective nature of the study, patient informed consent or ethical statement was not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2017;28:iv1-iv21. [Crossref]
- Rosen JE, Keshava HB, Yao X, et al. The Natural History of Operable Non-Small Cell Lung Cancer in the National Cancer Database. Ann Thorac Surg 2016;101:1850-5. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary. Chest 2013;143:7S-37S. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915-24. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Charloux A, Quoix E. Lung segmentectomy: does it offer a real functional benefit over lobectomy? Eur Respir Rev 2017;26:170079. [Crossref] [PubMed]
- Sato M, Murayama T, Nakajima J. The AMAGAMI technique: an easy technique to achieve precise stapling in thoracoscopic segmentectomy. J Thorac Dis 2019;11:276-9. [Crossref] [PubMed]
- Sun Y, Zhang Q, Wang Z, et al. Is the near-infrared fluorescence imaging with intravenous indocyanine green method for identifying the intersegmental plane concordant with the modified inflation-deflation method in lung segmentectomy? Thorac Cancer 2019;10:2013-21. [Crossref] [PubMed]
- Hennon M, Landreneau RJ. Role of Segmentectomy in Treatment of Early-Stage Non-Small Cell Lung Cancer. Ann Surg Oncol 2018;25:59-63. [Crossref] [PubMed]
- Migliore M, Fornito M, Palazzolo M, et al. Ground glass opacities management in the lung cancer screening era. Ann Transl Med 2018;6:90. [Crossref] [PubMed]
- Wolf AS, Richards WG, Jaklitsch MT, et al. Lobectomy versus sublobar resection for small (2 cm or less) non-small cell lung cancers. Ann Thorac Surg 2011;92:1819-23; discussion 1824-5.
- Cao J, Yuan P, Wang Y, et al. Survival Rates After Lobectomy, Segmentectomy, and Wedge Resection for Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;105:1483-91. [Crossref] [PubMed]
- Shapiro M, Weiser TS, Wisnivesky JP, et al. Thoracoscopic segmentectomy compares favorably with thoracoscopic lobectomy for patients with small stage I lung cancer. J Thorac Cardiovasc Surg 2009;137:1388-93. [Crossref] [PubMed]
- Filosso PL, Guerrera F, Falco NR, et al. Anatomical resections are superior to wedge resections for overall survival in patients with Stage 1 typical carcinoids†. Eur J Cardiothorac Surg 2019;55:273-9. [Crossref] [PubMed]
- Rahouma M, Kamel M, Narula N, et al. Role of wedge resection in bronchial carcinoid (BC) tumors: SEER database analysis. J Thorac Dis 2019;11:1355-62. [Crossref] [PubMed]
- Dansk Lunge Cancer Register 2017. Available online: årsrapport_2017-netudgave.pdfhttps://www.lungecancer.dk/wp-content/uploads/2019/05/DLCR_
- Seder CW, Wright CD, Chang AC, et al. The Society of Thoracic Surgeons General Thoracic Surgery Database Update on Outcomes and Quality. Ann Thorac Surg 2016;101:1646-54. [Crossref] [PubMed]
- Nomori H, Ohba Y, Shibata H, et al. Required area of lymph node sampling during segmentectomy for clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;139:38-42. [Crossref] [PubMed]
- Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041-5. [Crossref] [PubMed]
- Keenan RJ, Landreneau RJ, Maley RH, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33; discussion 228-33. [Crossref] [PubMed]



