Robotic segmentectomy: indication and technique
Introduction
Segmentectomy is becoming more popular as surgical approach in Thoracic Surgery, and, thanks to improvement of Imaging Technique and screening programs, more cancers are detected at early stage and require sublobar resections. In light of the recent results of the Nelson study, that confirmed a significant mortality reduction using Low-dose CT scan, lung cancer screening will be implemented soon in Europe with more than 70% of screening cancers (LC) detected in stage I–II, and a mean size of the tumor at diagnosis of 15 mm (1).
Segmentectomy for lung cancer was first described in 1973 by Jensik et al. (2), but due to the technical complexity and high risk of prolonged air-leaks it was not well accepted by surgeon at the very beginning. Moreover, a randomized trial of 1995 comparing limited resection to lobectomy in T1 N0 disease showed a significant increase in local recurrence in the limited resection group (3). However, critics were moved against these results: tumor >3 cm, usually associated to higher risk of nodal and distant metastasis, were included in this study. In addition, almost a third of patients in the limited resection group received wedge resection, a type of surgery that is usually less effective in terms of safe margins and does not provide for radical lymph node dissection compared to anatomical segmentectomy.
A large number of studies gave us positive oncological results: patients with T1N0 tumors ≤2 cm that underwent segmentectomy showed a 5 years overall survival comparable to standard lobectomy and a local recurrence rate significantly inferior compared to larger tumors (4,5).
The main limitation of those studies is the retrospective analysis of data, but the ongoing lobectomy versus segmentectomy prospective randomized trials (CALGB 140503 and JCOG0802) in patients with <2 cm peripheral lung cancer will provide objective information about sublobar anatomical resections (6,7).
Meanwhile, according to most surgical guidelines, indications to perform segmentectomy are as follow: tumor <2 cm where segmentectomy achieve >2 cm of parenchymal safe margin; stage I patients with poor lung function; multifocal lung cancer; selected recurrent tumor after major lung resection; no regional lymph node disease, age >75 years (8,9). In addition, according to our data, screening cancers showed a great variability in terms of Volume Doubling Time (VDT), ranging from 10 to 2,000 days and VDT seems to be a prognostic factor with a relationship to mortality rate in lung cancer disease. Patients with fast growing lung cancer (<400 days VDT) had a higher specific-mortality compared to slow-growing (400–600 days VDT) and indolent (>600 days VDT) (10). Thus VDT should be included among the key prognostic factors when surgeons plan the extension of surgical resection together with SUV at PET scan, size, density, position of the lesions.
In this scenario, it’s clear how traditional open lobectomy may lead to an overtreatment for patients with very initial tumors, as those that typically are found during LC screening, and how minimally invasive segmentectomy seems to be the proper and adequate proposal.
Among the available minimally invasive approaches, manual video-assisted thoracoscopic surgery (VATS) has been demonstrated to be a feasible and safe approach in order to achieve an oncologically correct procedure with reduced postoperative complications and faster recovery (11). However, complex anatomical segmentectomy can be very challenging using traditional manual VATS and more difficult compared to standard lobectomies, so only centers at very high volume allowed surgeons to reach appropriate surgical skills. At this regards robotic technologies come to help with 3D-vision and better dexterity, making operation much easier to perform (12). Moreover, the administration of intravenous indocyanine green (IV-ICG) with robotic camera shifted to infrared light makes the identification of segmental planes easier and precise (13) as described in our technical paper in 2014 (14). A phase II cohort trial evaluated safety, feasibility and reproducibility of IV-ICG for intersegmental plane identification during robotic surgery. Two thoracic surgeons where asked to identify the predicted segmental plane and afterward ICG was injected to map the real plane, resulting in an average increase of 2.4 cm in safe margin compare to the intersegmental plane predicted by surgeons (15). In our experience the use of ICG allowed a clear identification of the intersegmental plane, thus ensuring an appropriate safe margin around the tumor even if manual palpation wasn’t possible (16).
Surgical technique
Preoperative study
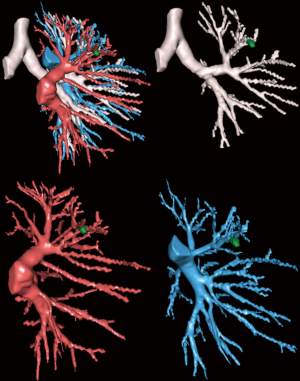
Preoperative evaluation includes chest CT and PET/CT scan, cardiologic and pulmonary function tests along with blood sample. Surgery is minutely planned with the use of MPR (Multi Planar Reconstruction) and 3D rendering technologies to better locate the lesion. Moreover, segmental branches may frequently vary and a better understanding of anatomy is needed using a closed chest approach. An accurate acquisition of vessels can be achieved by using Multidetector Computed Tomography (MDCT) angiography with volume-rendering 3D reconstruction. However this method does not allow the surgeon to focus on single anatomical structure one at a time and manipulate the 3D model, furthermore it’s a time-consuming protocol (17). Thanks to the improvements of technology, processing application like Osirix® includes now a free 3D modelling software (Autodesk Meshmixer) that makes easier to create preoperative models. It’s also possible to share anonymously the dicom files on online platform, like Visible Patients™, that generates interactive 3D modesl based on surgeon requests (Figure 1).
In our experience, Osirix® is a very good software for semi-automatic segmentation of Dicom data and Meshmixer offers the chance to create a personalized 3D model which can be exported in Standard Triangle Language (STL) for 3D printing, but requires good knowledge of Computer-Aided Design (CAD), is time-consuming for the surgeon and is an operator-dependent technique. It is available in a free (not CE labeled and FDA clearend) and a MD version. It is possible to buy a monthly or annual subscription and the price is based on the number and duration of license.
On the other hand Visible Patients™ is a ready-to-use service and it’s only needed to send the dicom files with a brief description of the type of surgery, allowing the society to create an interactive three dimensional model for the surgeon. However, there is a unitary price for every 3D model and is possible to visualize it only using a dedicated software from the same society.
Operating room setting
Surgical equipe position and instrumentation is similar to conventional VATS. All procedures are achieved under general anesthesia with double-lumen intubation. The patient is positioned in lateral decubitus with hip flexed and pelvis secured to operating table, thus giving stability all along the operation. The cart of XI Robotic System is usually positioned facing the ventral side of the patients and 4 arms are used.
Port placement
Two different groups of robotic technique and ports placement have been described, based on the presence or absence of utility incision and CO2 insufflation (18): RAL 4 (robotic assisted lobectomy) was described by Park/Veronesi and is characterized by 4 arms and 4 incisions. A 3 cm incision, the utility port, is made at IV-V intercostal space (ICS) anteriorly, the camera port is located at the VII–VII intercostal space (ICS) in the mid-axillary line, through which a 30° camera is inserted. On the left side, the camera port is positioned more laterally in order to avoid interferences with the heart. A supplementary 8 mm incision is crafted at VIII ICS on the tip of the scapula line. The fourth posterior incision is made in the auscultatory triangle. No CO2 insufflation is usually needed except for obese patients, high diaphragm or hyperinflated lung (19,20).
On the other hand, the CPRL technique does not use the utility incision at the beginning to benefit of the CO2 insufflation. Dylewski described a technique with 0° camera at the V-VI ICS above the major pulmonary fissure, plus two other ports on the same ICS (for a total number of 3 arms or CPRL-3). An additional 8 mm port is crafted at the end of the XI rib, passing through the X ICS. This access serve as passage for suction and stapler, lately will be enlarged for the extraction of specimen. Given the lower position, CO2 insufflation allow to lower the diaphragm thus improving movements and vision (21).
Cerfolio improved this technique by the use of a supplementary arm (CPRL-4) and positioning all the ports along the VII ICS, ranging from the mid-axillary line to two-three centimeters before the spinous processes. The space between the accesses was 9–10 cm when the Si is used. The supplementary port is positioned 2–3 ribs lower, CO2 and 0° camera is used (22).
S1 Segmentectomy (apical), right upper lobe
Dissection begins from the anterior aspect of the hilum until the exposure of V1 and the distal branches V1a-V1b for the apical segment of right upper lobe. The vein is then ligated either using clips or linear stapler. Then, anterior trunk is visualized superiorly and A1 is identified as the most cephalad branch, which in most of cases arise with A3. Again, it is ligated and divided after sparing the recurrent A2 small branch. Posteriorly the B1 for the apical segment appears. It is isolated and divided as well with cut with endostapler.
S2 Segmentectomy (posterior), right upper lobe
The approach of the major fissure is the first step in this operation, and consists of opening the intersection between the fissure and expose the main pulmonary artery in order to identify the junction between target A2 branch and A6 that must be spared. To better visualize vascular structures, inter-lobar lymph nodes must be excised. After ligation of A2 with clips or linear stapler, the B2 is located alongside. Transection of this bronchus exposes V2 which is then isolated and resected, allowing the removal of specimen, after transection of the parenchima with staplers.
S3 Segmentectomy (anterior), right upper lobe
First step consists of opening the mediastinal pleura over the hilum, starting from middle lobe vein to the anterior trunk and exposing the V3 for the ventral segment of right upper lobe. The vein then is ligated and transected using clips or stapler. More anteriorly, A3 originates from Boyden trunk along with A1 but in rare occasions they arise separately from main pulmonary artery. After ligation of the anterior segmental artery, B3 is exposed and closed by apposition of stapler. Completion of intersegmental plane with multiple fires of linear stapler allows the removal of specimen.
S1+2+S3 Segmentectomy (tri-segmentectomy), left upper lobe
Section of mediastinal pleura upon the left upper vein and removal of Station 5 lymph-node are crucial moments in the tri-segmentectomy. V1+2 and V3 are ligated and transected with clips or stapler, exposing the anterior trunk (A1+2 and A3 vessels). Usually a lymph node is found between the A1+A2 and the vein and should be removed before encycle the arteries. After section of this branch, the B1+2 and B3 stems from the anterolateral surface of the left main bronchus and can be isolated and resected with stapler. Inflation of the parenchima allow to check patency of lingular bronchus before firyng the stapler. Afterwards, a supplementary arterial branch behind the bronchial stump can be often found. As usual, it can be ligated and divided with clips or linear mechanical suture. Transection of the fissure between apical segments and lingula is the final step before specimen extraction.
S4+S5 Segmentectomy (lingulectomy), left upper lobe
As for the tri-segmentectomy, also the lingulectomy begins with removal of station 5 lymph-nodes and incision of mediastinal pleura over the hilum. V4+5 usually arise from a common trunk that must be isolated from venous branches to the apical segments of left upper lobe. Lingular branches are closed with clips, sutures or staplers and then the fissure is approach to identify the artery expose the A4+5 branches. Subsequently, is possible to complete the anterior fissure using staplers. The artery is isolated and ligated, showing off the B4+5 just behind it. After suturing the segmental bronchus, it is possible to separate the lingular parenchima from apical segments by multiple fires of stapler.
S6 Segmentectomy (upper), right or left lower lobe
Surgery begin with division of the pulmonary ligament up to the inferior pulmonary vein, after which the posterior fashion of the hilum is exposed and the V6 is identified and divided with clips or stapler. Next, division of the pulmonary fissure allow the identification posteriorly of A6 which is isolated and ligated. Completion of the posterior part of great fissure, exposes the B6 and allows the isolation and section with stapler. The parenchima is transected with staplers after injection of ICG.
S7-10 Segmentectomy (basilar), right or left lower lobe
After division of pulmonary ligament up to the inferior pulmonary vein, careful dissection reveals the V7-10 and V6, the latter must be spared. After isolation and division of the targeted segmental vein, parietal pleura is opened over the intersection of the fissures and A7-10 is revealed. Again, is essential to individuate and preserve arteries for middle lobe and apical segment of inferior lobe. Ligation and section of the segmental artery with clips or stapler expose the B7-10 bronchus, usually surrounded by lymph nodes (station 11) which are removed in order to facilitate isolation and closure of the bronchus. Lastly, basilar segments are separated from the rest of the lobe through the use of stapler and then removed.
Lymphnodes dissection
No swipe in instrumentation and trocars position is needed, the fourth arm in used to retract or move the lung and the assistant introduces suction from the utility incision in order to remove blood or smoke and occasionally provide retraction. Usually, hilar lymphadenectomy is performed during dissection of the hilum. Removal of station 5 (Aorto-Pulmonary window) at the very beginning facilitate the isolation of branches for left upper lobe, either for lingulectomy or trisegmentectomy procedures. Radical lymph node dissection includes en bloc removal of lymph nodes with the fatty tissue around, according to standard guidelines including at least three mediastinal stations including station 7 (23).If any of hilar or mediastinal lymph nodes appear enlarged >1 cm, it is preferred to execute a frozen section in order to rule out any chance of nodal metastasis.
Bronchus identification
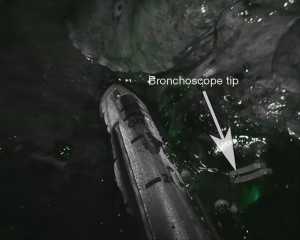
Intraoperative individuation of segments is possible through the use of Near Infra-Red (NIR) technology available on the Da Vinci system, commercially called Firefly™, along with the bronchoscope (Figure 2). After individuation of the bronchus, the anesthesiologist is asked to introduce the bronchoscope inside the double-lumen tube and reach the target segment. Then, the operator switch to Firefly™ mode in order to see the tip of the instrument as a lightish green that appear inside the airways.
Intersegmental plane identification
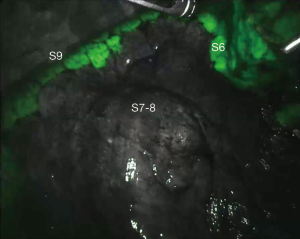
After closure of the artery for the targeted segment, anesthesiologist injects 6–8 mL of diluited ICG (2.5 mg/10 mL) followed by a push of 10 mL saline solution. Firefly™ mode is activated and after 30–40 seconds a green glow appears from mediastinal and lung tissue, reaching the maximum intensity within a minute and fading slowly afterwards. The unperfused segment appears in black and white, with a clear limit from the rest of the lobe which is green colored. Using bipolar forceps equipped on one arm, the operator can easily mark the intersegmental plane by spot coagulation (Figure 3). Afterwards, normal light source is activated and assistant can introduce linear stapler following the marks on the parenchyma.
Perioperative outcome
Duration of surgery vary from 84 to 240 minutes, depending on surgeon experience, volume of procedures and complexity of the segment (24). In a recent metanalysis, mean chest tube duration was reported to be 4.1 days, with a mean hospital stay of 4.89 days. Perioperative morbidity affected 27.5% of patients and 30 days mortality was about 0.7% (25).
Cost analysis
Cost of new technology is the main limitation to wide spreading. Initial cost is 1 million dollars, plus the cost of the robotic arms that have limited life-cycles (usually 10). Plus, there are no differences in regional refund between robotic, VATS or open surgery (19). In our previous experience we calculated the costs of robotic lobectomy and found that compared to vats and open there was an average increase cost of 12% mainly because of robotic disposable, drapes and longer duration of procedure. On the other hand, mean hospital stay was inferior (4 days versus 5 in VATS and 6 in open surgery) and personnel cost was lower in robotic procedures (26). Another study from Ghulam et al. compared the overall costs between RATS and VATS segmentectomy. There were no significant differences in the 2 groups regarding patient characteristics and surgical outcome, but the final cost of RATS procedure was lower compared to VATS mainly because of shorter length of stay (27).
Conclusions
Introduction of LDCT lung cancer screening make possible to detect lung cancer at a very early stage, but also opened the door to new entities like multifocal ground glass opacity, for which a standard still doesn’t exist. Thus with the widespreading of lung cancer screening in Europe sub-lobar resection will probably become the preferred surgical options for many patients. Segmentectomy has the potential to give the same oncologic results with better preservation of respiratory function, but more validation is needed and the two ongoing randomized trials will tell us whether or not this statement will be true (6,7). VATS is a feasible and safe approach and has several advantages over open surgery, but also comes with some important limitations: long learning curve, rigid instruments with counterintuitive movements, fulcrum effect and tremor amplification. All those limitations can be overcome with the use of robotic technology, making easier and safer minimally invasive segmentectomies. Higher cost is the main limitation nowadays but more studies are required to verify sustainability as for robotic lobectomies. it is still possible to cover the expense with the reimbursement from Health Public System in Italy (26). The Intuitive Surgical is the first society that produced CE approved surgical robot and had the monopoly for twenty years, but recently other companies entered the market thus generating competition and lowering of supplies prices. The intravenous Indocyanine Green administration is a safe and easy method to identify the intersegmental plane and may help to achieve an adequate safe margin compared to the existing technique (inflation-deflation and vice versa).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mario Nosotti, Ilaria Righi and Lorenzo Rosso) for the series “Early Stage Lung Cancer: Sublobar Resections are a Choice?” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.02.53). The series “Early Stage Lung Cancer: Sublobar Resections are a Choice?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Osarogiagbon RU, Veronesi G, Donington J, et al. Early-Stage NSCLC: Advances in Thoracic Oncology 2018. J Thorac Oncol. 2019;14:968-78. [Crossref] [PubMed]
- Jensik RJ, Faber LP, Milloy FJ, et al. Segmental resection for lung cancer: a fifteen-year experience. J Thorac Cardiovasc Surg 1973;66:563-72. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller?. Ann Thorac Surg 2001;71:956-60; discussion 961. [Crossref] [PubMed]
- Cao C, Chandrakumar D, Gupta S, et al. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer 2015;89:121-32. [Crossref] [PubMed]
- Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915-24. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [Crossref] [PubMed]
- Mun M, Kohno T. Eff icacy of thoracoscopic resection for multi-focal bronchioloalveolar carcinoma showing pure ground-glass opacities of 20 mm or less in diameter. J Thorac Cardiovasc Surg 2007;134:877-82. [Crossref] [PubMed]
- Veronesi G. Estimating Overdiagnosis in Low-Dose Computed Tomography Screening for Lung Cancer: A Cohort Study. Ann Intern Med 2012;157:776-84. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for earlystage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8.
- Veronesi G. Robotic lobectomy and segmentectomy for lung cancer: results and operating technique. J Thorac Dis 2015;7:S122-30. [PubMed]
- Ferrari-Light D, Geraci TC, Sasankan P, et al. The Utility of Near-Infrared Fluorescence and Indocyanine Green During Robotic Pulmonary Resection. Front. Surg 2019;6:47. [Crossref] [PubMed]
- Pardolesi A, Veronesi G, Solli P, et al. Use of indocyanine green to facilitate intersegmental plane identification during robotic anatomic segmentectomy. J Thorac Cardiovasc Surg 2014;148:737-8. [Crossref] [PubMed]
- Mehta M, Patel YS, Fahim C, et al. Near-infrared mapping with indocyanine green is associated with an increase in oncological margin length in minimally invasive segmentectomy. J Thorac Cardiovasc Surg 2019;157:2029-35. [Crossref] [PubMed]
- Novellis P, Bottoni E, Alloisio M, et al. Robotic-assisted pulmonary segmentectomies. J Vis Surg 2018;4:166. [Crossref]
- Gossot D1, Lutz J1, Grigoroiu M, et al. Thoracoscopic anatomic segmentectomies for lung cancer: technical aspects. J Vis Surg 2016;2:171. [Crossref] [PubMed]
- Casiraghi M, Galetta D, Spaggiari L. Robotic assisted lobectomy and lymphadenectomy “different approaches”. Shanghai Chest 2018;2:17. [Crossref]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Ninan M, Dylewski MR. Total port-access robot-assisted pulmonary lobectomy without utility thoracotomy. Eur J Cardiothorac Surg 2010;38:231-2. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Toker A, Ayalp K, Uyumaz E, et al. Robotic lung segmentectomy for malignant and benign lesions. J Thorac Dis 2014;6:937-42. [PubMed]
- Liang H, Liang W, Zhao L, et al. Robotic Versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-analysis. Ann Surg 2018;268:254-9. [Crossref] [PubMed]
- Novellis P, Bottoni E, Voulaz E, et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: comparison of costs and outcomes at a single institute. J Thorac Dis 2018;10:790-8. [Crossref] [PubMed]
- Musgrove KA, Hayanga JA, Holmes SD, et al. Robotic versus Video-AssistedThoracoscopic Surgery Pulmonary Segmentectomy: A Cost Analysis. Innovations (Phila) 2018;13:338-43. [Crossref] [PubMed]