Chemotherapy versus tyrosine kinase inhibitor in EGFR unselected population advanced non-small cell lung cancer still matter of debate?—An update incorporating the DELTA trial data
At present, for the treatment of second line advanced non-small cell lung cancer (NSCLC), three drugs are now available, pemetrexed, docetaxel and erlotinib (1-3). Docetaxel was registered in a superiority trial over placebo and it became the first second line approved at the beginning of this century, while erlotinib was licensed with a pivotal trial against placebo five years later (2).
In principle, erlotinib was conceived as an alternative drug in patients unfit for chemotherapy (2), however later it became an alternative option for all patients in second line advanced NSCLC.
In fact, several trials run after this registration comparing erlotinib or other EGFR-tyrosine kinase inhibitors (EGFR TKIs) versus chemotherapy and achieving similar results, with different expected toxicity.
However, in 2004 three groups at the same time discovered the presence of EGFR mutations opening the new era of the concept of targeted therapies (4-6). EGFR mutations were mainly in exons 19 and 21 and they were exquisitely sensitive to EGFR TKIs.
These mutations were typically more frequent in Asians, females and never smokers and further studies confirmed that when they were present the treatment with EGFR TKIs was clearly the treatment of choice also in first line.
The discovery of the presence of mutations in the EGFR genes and later of translocations in ALK gene as well as mutations and translocations in others genes (ROS1, BRAK, HER2, etc.) in NSCLC has allowed the introduction of targeted therapies directed against these genetic alterations and it became the proven possibility of creating the personalized medicine (7-10).
Nevertheless all these discoveries, all trials comparing erlotinib or other EGFR TKIs to chemotherapy, run independently from EGFR mutational status, giving controversial results on the role of these drugs in patients with wild-type EGFR.
At least four independent trials, mainly in Italy (TAILOR, PROSE) and in Asia (DELTA, CTONG), tried to properly address this issue on the selection. TAILOR randomized only patients with wild-type EGFR and it showed a superiority of docetaxel over erlotinib in terms of PFS, and close to the statistical significance in terms of overall survival (OS) (11). PROSE identified a proteomic signature for which patients were not to be considered for EGFR TKIs (12). CTONG compared gefitinib over pemetrexed, again showing a superiority of chemotherapy in EGFR wild-type population (13). The recent publication of the results of a randomized phase III trial comparing erlotinib versus docetaxel (in second or third line) in patients with advanced NSCLC (DELTA trial) by Kawaguchi et al. (14), added another significant piece, confirming the results of the previous TAILOR trial (Table 1).
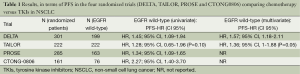
Full table
The DELTA trial was a multicenter randomized trial that included Japanese patients with advanced NSCLC (stage IIIB or IV), a previous history of chemotherapy (one or two regimens, with one at least containing platinum. Patients who previously received either docetaxel or erlotinib were excluded from the study. A total of 301 patients were randomized to receive erlotinib (150 pts) at the dose of 150 mg per day or docetaxel (151 pts) as 1 h infusion of 60 mg/sqm every three weeks (the dose of docetaxel was the one approved in Japan). The primary endpoint of the study was PFS while the secondary endpoints were OS, response rate safety and subgroup analysis in EGFR wild-type and mutant tumors. The patients were enrolled independently from the presence of EGFR mutations or wild-type.
The analysis performed showed that no differences in PFS were seen between erlotinib and docetaxel in the whole population. The median PFS was 2.0 months for erlotinib and 3.2 months for docetaxel. These results are consistent with previous results published in unselected patients.
In the DELTA trial EGFR status was determined in 255 patients (roughly 85% of the total randomized population). Of these, 199 had wild-type EGFR and 51 activating mutation of EGFR. Analysis in wild-type and mutant tumors showed that docetaxel was superior to erlotinib in the wild-type EGFR population with a PFS of 2.9 and 1.3, respectively. As expected the situation was opposite in the mutants EGFR population with erlotinib showing a longer PFS than docetaxel (9.3 vs. 7.0, respectively). The differences were not seen in OS, mostly due to the fact that patient in the DELTA trial were allowed to cross-over therapy (and indeed 40% of the patients received cross-over treatments).
Another interesting result was, consistent with those reported in the TAILOR trial, also in never smokers patients, no superiority of erlotinib was observed. In fact the HR was 1.32 (95% CI, 0.83-2.23) favouring chemotherapy.
The main characteristic of DELTA was that patients could also be enrolled with EGFR not determined. When patients were unselected, although the point estimate of the HR was slightly in favour of chemotherapy, no clear difference between the two regimes was observed. In case of EGFR mutated patient, despite the small number, erlotinib clearly performed better. In the case of patients harbouring an EGFR wild type, DELTA curved tallied TAILOR. Chemotherapy was superior in terms of PFS, but not in terms of OS. It is worthy to be highlighted that the benefit in terms of OS in TAILOR was more enhanced because, cross-overs third lines were not allowed. In fact, patients with EGFR wild-type tumors who were treated with docetaxel and did not receive subsequent therapy had a trend toward longer OS when compared with patients treated with erlotinib (HR, 1.79; 95% CI, 0.95-3.35; P=0.06).
Do the results mean that chemotherapy remains the only option for patients with wild-type EGFR disease? In our opinion after excluding other abnormalities potentially targetable (gene rearrangements in ALK, ROS1, RET, and NTRK1; mutations in HER2 and BRAF; and amplification of MET) the answer is yes. In the “wild-type” patients chemotherapy is preferable.
The results altogether, analyzed in a broader context clearly demonstrate, as was with the TAILOR trial that in patients with wild-type EGFR docetaxel is superior to target agents erlotinib.
Unfortunately, the sad conclusion of all these trials is that docetaxel is increasing in this population only two months the survival and this is very far from the results that we would like to achieve for our patients.
Nowadays, at least three lines of research are present giving hopes for the future. One is the rapid discovery of new abnormalities potentially targetable, the second is angiogenesis. In 2014 two drugs gave positive results for this setting, nintedanib and ramucirumab (15,16).
Finally, new promising drugs targeting the immune checkpoints are being tested, for which preliminary data suggest a change of the paradigm.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-103. [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [PubMed]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. [PubMed]
- Han SW, Kim TY, Hwang PG, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol 2005;23:2493-501. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [PubMed]
- Torjesen I. Large personalised medicine trial in lung cancer heralds new research partnership. BMJ 2014;348:g2837. [PubMed]
- Garassino MC, Platania M, Broggini M, et al. To target or not to target, that is the question. J Clin Oncol 2013;31:1254. [PubMed]
- Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013;14:981-8. [PubMed]
- Gregorc V, Novello S, Lazzari C, et al. Predictive value of a proteomic signature in patients with non-small-cell lung cancer treated with second-line erlotinib or chemotherapy (PROSE): a biomarker-stratified, randomised phase 3 trial. Lancet Oncol 2014;15:713-21. [PubMed]
- Zhou Q, Cheng Y, Yang JJ, et al. Pemetrexed versus gefitinib as a second-line treatment in advanced nonsquamous nonsmall-cell lung cancer patients harboring wild-type EGFR (CTONG0806): a multicenter randomized trial. Ann Oncol 2014;25:2385-91. [PubMed]
- Kawaguchi T, Ando M, Asami K, et al. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol 2014;32:1902-8. [PubMed]
- Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. [PubMed]
- Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014;15:143-55. [PubMed]


