Malignant pheochromocytoma with lung metastasis after right adrenalectomy for pheochromocytoma eleven years ago
Introduction
Pheochromocytoma is a catecholamine-secreting tumor presenting with high blood pressure. When there is no chromaffin tissue in previously noted tumor existence area, it is diagnosed as malignant pheochromocytoma (1). Although malignancy rate of these tumors are approximately 10% (1), their overall 5-year survival rate is less than 60% (2). Malignant pheochromocytoma usually has poor prognosis. However, accurate diagnosis and proper surgical resection might yield good clinical outcome.
Case report
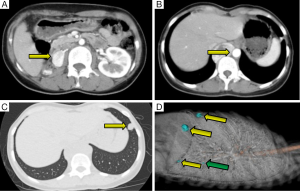
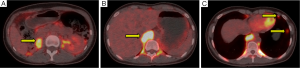
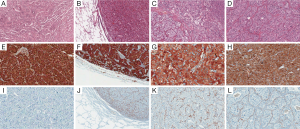
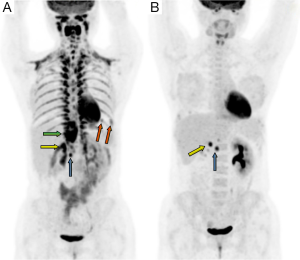
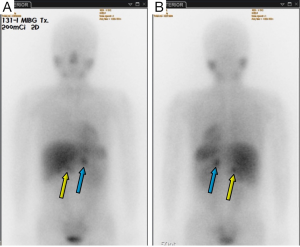
A 30-year-old woman with palpitation was found with retroperitoneal tumor on her regular checkup. Eleven years ago, she had systolic blood pressure up to 280 mmHg. Thus, she went through contrast-enhanced abdominal-pelvic computed tomography (CT) which revealed right adrenal pheochromocytoma. She underwent right adrenalectomy to remove the tumor. After the surgery, she ceased her regular follow up because she felt that her symptoms had subsided. At her recent admission for her checkup, she only had intermittent palpitation with normal blood pressure. Considering the patient was a young female and she had previous adrenalectomy operation, an endocrinology professional recommended her genetic tests for multiple endocrine neoplasia type 2 (MEN 2), Von Hippel-Lindau disease, neurofibromatosis, and familial paraganglioma. However, she and her family members refused to have tests because they didn’t have a history of associated symptoms. In addition, the cost was too expensive. In preoperative CT, there were four nodules at less than 1 cm in the left lung (one in upper lobe, three in lower lobe), a 4.6 cm high attenuated mass in the right retrocrural area at diaphragm level, and a 2.8 cm high enhanced mass at the previous right adrenalectomy site (Figure 1). Laboratory findings were: plasma metanephrine at 0.03 mg/day, plasma normetanephrine at 20.92 mg/day, serum epinephrine at 315.83 pg/mL, and serum norepinephrine at 1,501.61 pg/mL (Table 1). Even if her biochemistry findings did not suffice the diagnostic criteria for pheochromocytoma or paraganglioma, she was highly suspected of pheochromocytoma based on imaging studies (Figure 2). To rule out recurrent pheochromocytoma at the previous right adrenalectomy site, we performed surgical resection with the general surgery team. Preoperative CT-guided wire hooking was performed on the smallest nodule in segment 6 of the left lung. During the operation, alpha blocker was pre-medicated to control blood pressure for 6 weeks. In right lateral decubitus position, video-assisted thoracoscopic multiple wedge resection of left lung was done by our surgical staff. After we changed the position of the patient to supine, right thoracotomy along the 9th intercostal space was done. Retrocrural mass resection was followed. The hypervascular pulsating mass at T10-L1 level was 6.5 cm × 4.5 cm in size (Figure 3). In the same field, the general surgery team opened retroperitoneum to perform complete resection at the adrenalectomy site. The retroperitoneal mass was 3 cm × 2.5 cm in size and well-capsulated. The entire surgical procedure was finished without difficulties. Histopathological diagnoses of all specimens were pheochromocytoma (Figure 4). After the surgery, plasma normetanephrine and serum epinephrine were normalized (Table 1). Postoperative PET image showed that the uptake amount and size of previous noted hypermetabolic nodular lesions were decreased. However, such lesions had not been disappeared. Moreover, we noticed residual hypermetabolic lymph nodes at aortocaval and retrocaval area. We assumed that these lesions were residual masses and metastases. Changes of preoperative and postoperative maximum standardized uptake value (mSUV) in the adrenalectomy site, retrocrural, and largest two lung nodules were from 10.84 to 13.92, 17.55 to 0, and 5.65, 5.64 to 0, respectively (Figure 5). In postoperative 123iodine-metaiodobenzylguanidine (123I-MIBG) imaging, increased uptake of residual pheochromocytoma in right adrenalectomy site, retroperitoneal lymph nodes, and residual mass were observed (Figure 6). After one 131I-MIBG treatment at 200 mCi, further treatment was stopped due to pregnancy and delivery. Any sign of severe high blood pressure or symptom of paroxysmal palpitation was not present during the close monitoring period.


Full table
Discussion
Most pheochromocytomas are originated from chromaffin cells of the adrenal medulla. Approximately 10% of them are extra-adrenal paragangliomas containing chromaffin cells (3). It is difficult to diagnose malignant pheochromocytoma with histopathologic studies (4). Other methods of identifying malignancy from benign pheochromocytomas include pheochromocytoma of adrenal gland scaled score (PASS), Ki-67 index, HSP 90 (heat-shock protein 90), and activator of transcription 3 (STAT 3). However, their efficacy is still controversial (5-7).
Regardless of recurrence, malignant pheochromocytomas is defined as an occurrence of distant metastasis where chromaffin tissue is absent, not by local invasion (8). Typical sites of metastasis are bone, lung, liver, and lymph node (2,9). Malignancy rate of primary adrenal pheochromocytoma and sympathetic paraganglioma are 25% and 60%, respectively (10). Several literatures reported independent risk factors of malignancy, including the presence of succinated dehydrogenase subunit B (SDHB) mutation, multifocal and extra-adrenal location, size of the primary tumor (5 cm or larger), heavy tumor (250 g or greater), younger age, early onset postoperative hypertension, increased levels of plasma methoxytyramine, and higher plasma or urine metadrenalins (9-15). In our case, risk factors of malignant pheochromocytoma are were: young age (30 years old), paraganglioma larger than 5 cm, and extra adrenal gland location.
Although there is no clear symptom or laboratory finding for pheochromocytoma or paraganglioma, if such disease is suspected based on imaging studies, we can conclude malignancies base on previous operative history and risk factors because metastatic pheochromocytoma has very poor prognosis.
In our case, the mass at the previous right adrenalectomy site was considered as a recurrence of remnant tumor cell or spillage during manipulation of first right adrenalectomy. Moreover, posterior mediastinal mass in the retrocrural area at T10-L1 level could be a primary paraganglioma from nerve sheath because pheochromocytoma is a hypervascular solid mass. However, we cannot preclude the possibility of disseminated seeding through retroperitoneum from remnant tumor during previous operative manipulation.
Brennam et al. (16) reported that failure to find extra lesions at primary exploration will develop metachronous primary tumor in remnant adrenal or extra-adrenal chromaffin tumor due to disruption and implantation during previous operation. They also suggested that such failure in finding extra lesions might cause metastatic disease after surgical resection. In the case reported here, the origin of lung metastasis was unclear based on histopathological findings. It might be a hematogenous spread from either remnant retroperitoneal tumor or posterior mediastinal tumor. As mentioned above, paraganglioma has more frequent metastasis risk than pheochromocytoma. Therefore, the lung nodules in our patient might be metastases from paraganglioma.
Based on postoperative PET and MIBG studies, residual masses might be seeded from manipulation during previous operation. We thought that all four nodules in the lung and retrocrural mass were totally resected. However, since residual mass of adrenalectomy site in PET and MIBG was seen, suggesting incomplete radical resection. Moreover, unchanged mass of retrocaval and aortocaval area in PET suggested incomplete exploration. Characteristics of pheochromocytoma in the sympathetic chain and other area associated with chromaffin tissue will make complete resection difficult. We conclude that close observation of these residual tumors by MIBG study is crucial.
Due to the hormonal effect of catecholamine of pheochromocytoma during multiple site resections, we studied the patient’s unstable hemodynamic status. Fortunately, alpha blocker and beta blocker premedication more than six weeks made patient stable. After being diagnosed of malignant pheochromocytoma, some patients survived more than 20 years (17). However, prognosis of malignancy is generally poor, with less than 60% on 5-year survival rate (2). However, the survival rate of benign pheochromocytoma is 90% on 5-year survival rate (18). Classic symptoms of pheochromocytoma include headache, diaphoresis, and flushing (appear in 40% of all patients with pheochromocytoma). The rest do not show any specific symptoms (19). For that reason, the diagnosis of phemochoromycytoma is difficult. In addition, patients have poor prognosis. When risk factors of malignant as mentioned above are presented when the first episode starts, more careful follow up is needed. Noshiro et al. (20) have reported recurrence in more than 10 years after primary adrenalectomy for pheochromocytoma which is similar to our case. It was recommended that the follow up period should be at least 10 years from 1st operation or 1st diagnosis of pheochromocytoma.
Conclusions
When silent pheochromocytoma with biochemical inactivity is identified, we have to consider surgical resection instead of therapeutic modalities if risk factors of metastatic malignant pheochromocytoma are presented with suspicious radiologic findings. Surgery will lead to better outcome. Although we thought that we completed radical excision of all masses, there might be possibilities of recurrence or metachronous development along the chromaffin tissues. Therefore, we should follow up at least 10 years by MIBG study.
Here we report a patient who underwent right adrenalectomy eleven years ago developed multiple metastases at the previous right adrenalectomy site as well as retrocrural and lung lesions. In this case, we completely excised metastatic masses and closed observe patient on MIBG can without side effects.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Eisenhofer G, Bornstein SR, Brouwers FM, et al. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer 2004;11:423-36. [PubMed]
- Jimenez C, Rohren E, Habra MA, et al. Current and future treatments for malignant pheochromocytoma and sympathetic paraganglioma. Curr Oncol Rep 2013;15:356-71. [PubMed]
- Lenders JW, Eisenhofer G, Mannelli M, et al. Phaeochromocytoma. Lancet 2005;366:665-75. [PubMed]
- Hodin R, Lubitz C, Phitayakorn R, et al. Diagnosis and management of pheochromocytoma. Curr Probl Surg 2014;51:151-87. [PubMed]
- Thompson LD. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol 2002;26:551-66. [PubMed]
- de Wailly P, Oragano L, Radé F, et al. Malignant pheochromocytoma: new malignancy criteria. Langenbecks Arch Surg 2012;397:239-46. [PubMed]
- Tavangar SM, Shojaee A, Moradi Tabriz H, et al. Immunohistochemical expression of Ki67, c-erbB-2, and c-kit antigens in benign and malignant pheochromocytoma. Pathol Res Pract 2010;206:305-9. [PubMed]
- Thompson LD, Young WF, Kawashima A, et al. Malignant adrenal phaeochromocytoma. In: DeLellis RA, Lloyd RV, Heitz PU, et al. eds. WHO Classification of Tumors: Pathology and Geneticstumors of Endocrine Organs. Lyon, France: IARC, 2004:147-50.
- Plouin PF, Fitzgerald P, Rich T, et al. Metastatic pheochromocytoma and paraganglioma: focus on therapeutics. Horm Metab Res 2012;44:390-9. [PubMed]
- Ayala-Ramirez M, Feng L, Johnson MM, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab 2011;96:717-25. [PubMed]
- Eisenhofer G, Lenders JW, Siegert G, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer 2012;48:1739-49. [PubMed]
- Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab 2007;92:3822-8. [PubMed]
- Park J, Song C, Park M, et al. Predictive characteristics of malignant pheochrom-ocytoma. Korean J Urol 2011;52:241-6. [PubMed]
- Zelinka T, Musil Z, Dušková J, et al. Metastatic pheochromocytoma: does the size and age matter? Eur J Clin Invest 2011;41:1121-8. [PubMed]
- Feng F, Zhu Y, Wang X, et al. Predictive factors for malignant pheochromocytoma: analysis of 136 patients. J Urol 2011;185:1583-90. [PubMed]
- Brennan MF, Keiser HR. Persistent and recurrent pheochromocytoma: the role of surgery. World J Surg 1982;6:397-402. [PubMed]
- Yoshida S, Hatori M, Noshiro T, et al. Twenty-six-years' survival with multiple bone metastasis of malignant pheochromocytoma. Arch Orthop Trauma Surg 2001;121:598-600. [PubMed]
- Loh KC, Fitzgerald PA, Matthay KK, et al. The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): a comprehensive review of 116 reported patients. J Endocrinol Invest 1997;20:648-58. [PubMed]
- Guerrero MA, Schreinemakers JM, Vriens MR, et al. Clinical spectrum of pheochromocytoma. J Am Coll Surg 2009;209:727-32. [PubMed]
- Noshiro T, Shimizu K, Watanabe T, et al. Changes in clinical features and long-term prognosis in patients with pheochromocytoma. Am J Hypertens 2000;13:35-43. [PubMed]


