Clinical implications and future perspectives in testing non-small cell lung cancer (NSCLC) for anaplastic lymphoma kinase (ALK) gene rearrangements
Over the last decade different oncogenic drivers have been discovered in non-small cell lung cancer (NSCLC).
Among them, anaplastic lymphoma kinase (ALK) gene rearrangement, due to inversion or translocation of chromosome 2p, has become a new druggable target for anticancer therapy.
It has been a perfect example of synergism between molecular research, preclinical and clinical drug development.
Since ALK gene rearrangement has been identified as a new potential target in NSCLC (1), crizotinib, the first-in-class ALK inhibitor, received an accelerated approval by Food and Drug Administration (FDA) for the treatment of advanced or metastatic ALK-positive NSCLC in four years only.
To date, the European Medicines Agency (EMA) has approved crizotinib for the treatment of advanced, pre-treated ALK-positive NSCLC patients with a validated method (without a companion diagnostic test). More recently, the results of PROFILE 1014 study have been published (2). This trial confirmed that also untreated ALK-positive NSCLC patients significantly benefited from crizotinib treatment over the standard first-line chemotherapy. The advantage of crizotinib as compared to chemotherapy in terms of progression-free survival (PFS) was approximately of 4 months [10.9 versus 7.0 months, HR 0.45 (95% CI, 0.35-0.60); P<0.001]. Also objective response rate (ORR) was in favour of crizotinib (74% versus 45%, respectively; P<0.001).
After the publication of these data, it is plausible that also EMA will approve crizotinib in advanced ALK-positive NSCLCs, regardless of previous therapies.
Therefore, new urgent issues have to be solved. Among them, the prevalent topics have related to the available tumor samples, the several growing molecular tests required and the very low prevalence of ALK gene rearrangement in NSCLC patient populations.
In the majority of cases a diagnosis of NSCLC is obtained from small tumor biopsies or cytological smears rather than surgical samples (3).
According to the good clinical practice, in presence of a diagnosis of NSCLC the pathologist is requested to perform some immunohistochemical stains (TTF-1 and p40 or p63) and molecular DNA sequencing assays to distinguish non-squamous from squamous cell carcinoma and to detect activating epidermal growth factor receptor (EGFR) mutations, respectively.
In light of the approval of crizotinib therapy and to do not loose tumor tissue, it is now recommended to test ALK simultaneously with EGFR (+/− K-RAS) mutations.
The four proposed methods of testing ALK include fluorescent in situ hybridization (FISH), immunohistochemistry (IHC), reverse transcriptase polymerase chain reaction (RT-PCR) and DNA sequencing (3).
To date, FISH has been the test commonly used in clinical trials and it is the currently accepted method and approved by the FDA. Although FISH is the gold standard test, this method remains susceptible to several criticisms. In fact, FISH assay needs highly-specialized laboratories, expert pathologists and/or biologists and it is not an inexpensive method, making it not adequate as large-scale screening test. Furthermore, the cut-off for ALK-FISH positivity (≥15% of at least 50 nuclei expressing red and green split or isolated red signals) is arbitrary and lacking of any biological rationale (4). Finally, it may be unable to detect novel complex rearrangements (5).
IHC is the most common, affordable and available method in all pathology laboratories. It can be used successfully both on tumor biopsies and fine-needle aspiration cell blocks, as well as FISH. Given the lack of ALK protein expression in normal lung tissue, IHC seems the ideal technique in disclosing ALK-positive NSCLC. The main criticism of this method is related to the variability in terms of antibody sensibility and inter-observer agreement. Three antibodies are available, as follows: clones ALK1 (Dako), 5A4 (Novocastra/Leica) and D5F3 (Cell Signaling Technology/Ventana) (Figure 1). The first two clones need a four-tiered scoring system quoting tumor expression as 0, 1+, 2+ and 3+, according to the intensity of staining in ≥10% of tumor cells. The scoring of the last assay follows a two-tiered system recording tumor samples as ALK-negative and ALK-positive.
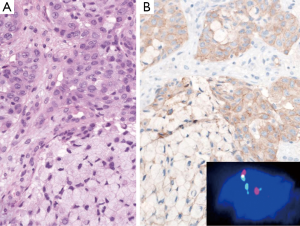
The sensitivity and specificity of IHC by using 5A4 antibody were 100% and 96%, respectively, when compared to FISH assay (6).
When compared to ALK1 antibody, D5F3 showed excellent sensitivity (100%) and specificity (99%), with a very high inter-observer agreement (κ=0.94) (7).
ALK gene rearrangement accounts for 5-6% of all NSCLC patients (8). Although ALK-positive NSCLCs are more frequently in young never-light smokers with adenocarcinoma wild-type for EGFR and KRAS mutations, there are no standard criteria to select patients for ALK gene rearrangements testing and all non-squamous NSCLC patients can be eligible for ALK FISH testing (9).
Therefore, it is essential a balanced use of the available tumor tissue with the aim to optimize diagnosis, treatment decision making and cost/effectiveness ratio.
For this purpose, an interesting strategy might be to design a specific diagnostic algorithm using a sensitive, specific and accurate ALK IHC assay as large-scale screening test.
At this regard, we read with very interest the work published by Blackhall and colleagues on the prevalence and prognostic impact of ALK positivity in resected lung adenocarcinoma (10). The study evaluated the prevalence of ALK positivity by IHC (5A4, Novocastra) and FISH (Vysis ALK break-apart FISH probe, Abbott Molecular, Des Plaines, IL, USA), as confirmatory test and its correlation with specific clinical features. Furthermore, it was designed to clear the prognostic impact of ALK positivity and to compare the two diagnostic methods in detecting ALK-positive cases. Surgical samples from 1,281 patients with lung adenocarcinoma were screened for ALK by IHC. Eighty of all samples (6.2%) were ALK IHC-positive (48 IHC 1+, 10 IHC 2+ and 22 cases IHC 3+) and were matched with 160 ALK IHC-negative cases (ALK IHC matched cohort). Among 80 ALK IHC-positive cases, twenty-eight (35%) were ALK FISH-positive (2.2% of all samples), whereas of the 160 ALK IHC-negative samples all were ALK FISH-negative.
The 28 ALK FISH-positive samples were matched with 56 ALK FISH-negative samples (ALK FISH matched cohort).
In the overall cohort, ALK IHC positive cases were significantly correlated with never-smoker status (P=0.017). After the comparison between ALK IHC/FISH positive and ALK IHC negative patients, age and smoking status were significantly associated with ALK FISH status. Finally, these data were also confirmed in ALK FISH positive patients when compared to ALK negative ones (P=0.0016 and P=0.001, respectively).
Within the entire patients cohort (1,281 pts), ALK IHC positive patients had a significant better outcome in terms of 5-year overall survival (OS) (66.6% versus 54.4%, P=0.012), relapse free survival (RFS) (57% versus 47.2%, P=0.018) and time to relapse (TTR) (68.6% versus 56%, P=0.015). These data were significant also at the multivariate analysis.
Within the ALK IHC matched cohort (240 pts), only 5-yr OS was significantly in favour of ALK IHC positive status (66.6% versus 57%; HR 0.54, P=0.02). The same results (5-yr OS: 73.4% versus 47%; HR 0.40, P=0.05) were reported also for ALK FISH positive patients within the ALK FISH matched cohort (84 pts).
Double positive (ALK IHC and FISH positive) patients had the highest probability of survival when compared to double negative patients (5-yr OS: 73.4% versus 54.4%, respectively; HR 0.42, P=0.022). Although this is the largest predominantly European data set to evaluate clinical outcome of ALK-positive patients with resected lung adenocarcinoma, the role of ALK positivity as favourable prognostic factor is still debated. As pointed out by the authors, there are no prospective data and several retrospective analyses showed discordant results (9). Finally, it is likely that the natural course of ALK-positive disease can be altered by treatment in the ALK inhibitors era (9).
As previously mentioned, the authors reported a good concordance between IHC and FISH testing, with a sensitivity and specificity for FISH after IHC of 35% and 100%, respectively. However, if we considered only samples with ALK IHC 2+ and 3+, the positive predictive value of ALK IHC testing increased up to 81.3% (26/32 samples), with a specificity of 99%. In the study, 13 cases had discordant IHC and FISH results: 9/13 were IHC 2+ and FISH negative, 2/13 were IHC 3+ and FISH negative and 2/13 were IHC 1+ and FISH positive.
Among the first nine cases, five was changed in IHC score (from 2+ to 1+) and the remainders were confirmed IHC 2+ and classified as RT-PCR positive (1 case), RT-PCR negative (2 cases) and RT-PCR not diagnostic (1 case). Among the two cases defined as IHC 3+/FISH negative, both were confirmed ALK negative by RT-PCR. The last two cases identified as IHC 1+/FISH positive were RT-PCR positive (1 case) and RT-PCR negative (1 case, with FISH positivity of 16%).
The study was well conducted and has confirmed that an IHC screening might be a reliable large-scale screening tool. In absence of standard criteria and predictive biomarkers to select patients for ALK screening, several previous studies have highlighted the very close concordance between IHC and FISH results, supporting the algorithmic use of ALK IHC in the evaluation of NSCLC (6,11-15). Given the absence of FISH positivity in IHC negative samples, IHC could become the primary screening tool for ALK-positive NSCLC, with only IHC 2+ and 3+ cases requiring confirmation by ALK FISH test. Tumor samples with IHC 1+ expression to retest by FISH should be restricted to cases with particular features. This pre-screening strategy would significantly reduce the cost of ALK testing and expedite its turnaround time. High-sensitivity protocols (particularly when using the clone D5F3 and OptiView amplification kit) make IHC entirely comparable to FISH results, basically not requiring FISH confirmatory testing in negative and strongly positive (score 3+) cases (16) (Figure 2).
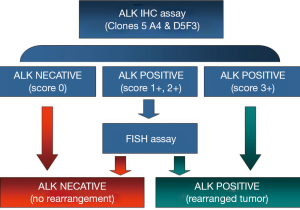
In conclusion, it has been demonstrated that the majority of patients with ALK-rearranged NSCLC benefit from crizotinib over chemotherapy (2,17). A part of this molecular subgroup continues to have an advantage from crizotinib beyond progression (i.e., cases with asymptomatic or isolated progression) (18). In presence of systemic progression at multiple sites, patients might receive ceritinib (19) or other second-generation ALK inhibitors able to overcome acquired resistance and to act against brain metastasis (20).
Taken into account these relevant clinical implications in a very small subgroup of NSCLC patients, specific predictive biomarkers for screening these patients are urgently required. IHC may be an effective, rapid and reproducible pre-screening assay, but this paradigm will be really effective only if highly sensitive ALK IHC protocols are developed and validated as predictive biomarkers for response to ALK inhibitors in a large patient cohort.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [PubMed]
- Hirsch FR, Wynes MW, Gandara DR, et al. The tissue is the issue: personalized medicine for non-small cell lung cancer. Clin Cancer Res 2010;16:4909-11. [PubMed]
- Camidge DR, Theodoro M, Maxson DA, et al. Correlations between the percentage of tumor cells showing an anaplastic lymphoma kinase (ALK) gene rearrangement, ALK signal copy number, and response to crizotinib therapy in ALK fluorescence in situ hybridization-positive nonsmall cell lung cancer. Cancer 2012;118:4486-94. [PubMed]
- Peled N, Palmer G, Hirsch FR, et al. Next-generation sequencing identifies and immunohistochemistry confirms a novel crizotinib-sensitive ALK rearrangement in a patient with metastatic non-small-cell lung cancer. J Thorac Oncol 2012;7:e14-6. [PubMed]
- Paik JH, Choe G, Kim H, et al. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non-small cell lung cancer: correlation with fluorescence in situ hybridization. J Thorac Oncol 2011;6:466-72. [PubMed]
- Mino-Kenudson M, Chirieac LR, Law K, et al. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res 2010;16:1561-71. [PubMed]
- Tiseo M, Gelsomino F, Bartolotti M, et al. Anaplastic lymphoma kinase as a new target for the treatment of non-small-cell lung cancer. Expert Rev Anticancer Ther 2011;11:1677-87. [PubMed]
- Capelletti M, Gelsomino F, Tiseo M. MET and ALK as targets for the treatment of NSCLC. Curr Pharm Des 2014;20:3914-32. [PubMed]
- Blackhall FH, Peters S, Bubendorf L, et al. Prevalence and clinical outcomes for patients with ALK-positive resected stage I to III adenocarcinoma: results from the European Thoracic Oncology Platform Lungscape Project. J Clin Oncol 2014;32:2780-7. [PubMed]
- Park HS, Lee JK, Kim DW, et al. Immunohistochemical screening for anaplastic lymphoma kinase (ALK) rearrangement in advanced non-small cell lung cancer patients. Lung Cancer 2012;77:288-92. [PubMed]
- Yi ES, Boland JM, Maleszewski JJ, et al. Correlation of IHC and FISH for ALK gene rearrangement in non-small cell lung carcinoma: IHC score algorithm for FISH. J Thorac Oncol 2011;6:459-65. [PubMed]
- McLeer-Florin A, Moro-Sibilot D, Melis A, et al. Dual IHC and FISH testing for ALK gene rearrangement in lung adenocarcinomas in a routine practice: a French study. J Thorac Oncol 2012;7:348-54. [PubMed]
- Just PA, Cazes A, Audebourg A, et al. Histologic subtypes, immunohistochemistry, FISH or molecular screening for the accurate diagnosis of ALK-rearrangement in lung cancer: a comprehensive study of Caucasian non-smokers. Lung Cancer 2012;76:309-15. [PubMed]
- Paik JH, Choi CM, Kim H, et al. Clinicopathologic implication of ALK rearrangement in surgically resected lung cancer: a proposal of diagnostic algorithm for ALK-rearranged adenocarcinoma. Lung Cancer 2012;76:403-9. [PubMed]
- Minca EC, Portier BP, Wang Z, et al. ALK status testing in non-small cell lung carcinoma: correlation between ultrasensitive IHC and FISH. J Mol Diagn 2013;15:341-6. [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [PubMed]
- Ou SH, Jänne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol 2014;25:415-22. [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [PubMed]
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119-28. [PubMed]


