
Thoracic positron emission tomography: 18F-fluorodeoxyglucose and beyond
Introduction
Since the early 2000’s, there have been many advancements in nuclear medicine which have become applicable to diagnosis and treatment of thoracic disorders. The most established application is positron emission tomography (PET). There is a broad base of evidence supporting the use of 18F-flurodeoxyglucose (FDG) PET combined with computed tomography (CT) (PET/CT) for evaluation of solitary pulmonary nodules and in the staging and follow up in patients with bronchogenic carcinoma. More recently, hybrid PET/MRI has shown promise in applications within these same diseases. At the same time, there has been development of novel radiotracers, chiefly those which target the somatostatin receptor, most commonly 68Ga-DOTATATE, which have revolutionized not only the imaging diagnosis in patients with neuroendocrine tumors such as bronchial carcinoid, but offer novel therapeutic options via targeted molecular therapies such as 177Lu-DOTATATE. The goal of this article is to provide an overview of current and emerging PET applications in thoracic neoplasms.
18F-FDG PET/CT
18F-FDG is a glucose analog that once taken up within a cell, is trapped within the cell and used as a surrogate marker for glucose metabolism. Glucose metabolism can be seen in a wide variety of situations including normal physiology, but can be increased in pathologic states such as infection or neoplasm. Initially developed in the early 2000’s, FDG PET/CT exam volumes have increased considerably over time, and these studies now comprise a cornerstone of oncologic imaging. Oncologic applications of 18F-FDG PET/CT include the evaluation of pulmonary nodules and the diagnosis and staging of bronchogenic carcinoma.
Pulmonary nodules
Pulmonary nodules are a frequent incidental finding when imaging the chest. Older literature estimates that there are 150,000 new pulmonary nodules detected per year in the United States, although this number has likely increased given increased usage of CT (1). The first approved indication for 18F-FDG PET/CT was for the characterization of solitary pulmonary nodules (2-5). A solitary pulmonary nodule (SPN) is defined as a round, solid, non-calcified lung lesion measuring <30 mm in mean diameter (6). Pulmonary nodules can be characterized as either solid or subsolid.
Solid pulmonary nodule
One of the most important factors for the evaluation of pulmonary nodules with 18F-FDG PET/CT is size. The spatial resolution of PET limits the evaluation pulmonary nodules less than 10 mm with older technology, such as a two-dimensional (2D) acquisition. Under this size, SUV can underestimate the true nodule metabolism, decreasing the negative predictive value (NPV). Advancements in PET imaging such as newer 3D and time-of-flight (TOF) acquisitions are now routine at most institutions; improving the NPV of small nodules and helping the characterization of nodules as small as 7–8 mm (7,8).
The definition of an 18F-FDG PET positive solid pulmonary nodule has changed throughout the years. The previously described SUV max “cut off” of 2.5 is no longer recommended (9). It is now recommended to correlate to internal controls with a SUV max less than blood pool being very likely benign. The accuracy of 18F-FDG PET/CT has proven to be to quite exceptional. In a recent meta-analysis reviewing 44 studies and over 2,800 nodules, the sensitivity was 95%, specificity 82%, PPV 91%, and NPV 90% (10). An example of FDG PET/CT used in the evaluation of a solitary pulmonary nodule is shown in Figure 1.
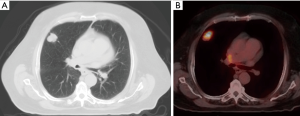
False positives for FDG avid SPN mainly include granulomatous processes (11). These can be broken down into infectious or inflammatory etiologies. Infectious diseases include bacterial, mycobacterial, and fungal diseases. The prototypical infection is histoplasmosis. Helpful discriminatory factors can include the so called Flip-Flop Fungus sign (Figure 2). In fungal infections, the FDG avidity of the SPN is less than the mediastinal/hilar lymphadenopathy. The opposite is true in malignancy, where the FDG avidity of the SPN is typically greater than the FDG avidity of the mediastinal/hilar lymphadenopathy (12).
Inflammatory processes that can have FDG avid SPNs include sarcoidosis, granulomatosis with polyangiitis, organizing pneumonia (Figure 3), or rheumatoid nodules. Helpful discriminatory factors are non-specific, but can include fluctuations in growth or a waxing and waning nature over time (13).
False negatives of SPN are also possible. This occurs in the setting of a malignancy that does not have increased glucose metabolism. The most well-known example is of carcinoid tumor, with a false negative rate of 85% (14). Consideration of alternative diagnoses and further evaluation with other tests such as 68Ga DOTATATE which will be described later should be considered in order to avoid false negative diagnoses. Additionally, metastatic disease from mucinous origins also generally have lower FDG uptake (15).
Subsolid pulmonary nodules
A pulmonary nodule that is not homogenous soft tissue attenuation is referred to as a subsolid pulmonary nodule. Subsolid pulmonary nodules pose many challenges for PET imagining associated with technical factors of image acquisition which can result in a perceived or quantitative decrease in FDG uptake (16). While a subsolid pulmonary nodule may be the manifestation of infectious or inflammatory etiologies, minimal and noninvasive adenocarcinoma lesions may also have this appearance.
Subsolid pulmonary nodules can be further classified as pure ground glass nodule or a part solid nodule. A pure ground glass nodule is defined as hazy increased attenuation in the lung that does not obliterate the bronchial or vascular margins. A part solid nodule consists of both pure ground glass and solid soft tissue attenuation (6). The utility of PET/CT in subsolid nodules was investigated by Chun et al. in 2008. This study demonstrated perhaps counterintuitively that in part solid nodules, the SUVmax was significantly higher in inflammatory lesions compared to malignant tumors. A threshold-value SUVmax of 1.2 predicted malignancy with sensitivity, specificity, accuracy, PPV and NPV of 62.1%, 80.0%, 70.4%, 78.3% and 64.5%, respectively. Furthermore, above an SUV max of 2.6, all lesions were inflammatory. For pure ground glass nodules, there was no statistical differences in SUV max between inflammation and malignancy (17). Ultimately, differentiation between infectious/inflammatory subsolid nodules and adenocarcinoma spectrum lesions remains challenging and may serve as a future area of investigation.
Bronchogenic carcinoma
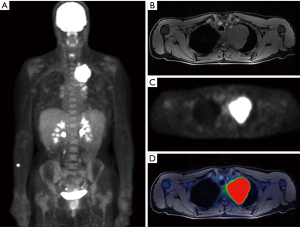
Bronchogenic carcinomas are traditionally characterized as either non-small cell lung cancer (NSCLC) or small cell lung cancer (SCLC). While all types of bronchogenic carcinoma are typically FDG avid (Figures 4,5), malignancies along the adenocarcinoma spectrum can be variable in their uptake.
Lung adenocarcinoma can have multiple imaging appearances including solid, pure ground glass, and part solid nodules. The solid type usually presents as a SPN where 18F-FDG PET/CT can be helpful as previously described. In the case of ground glass nodules, PET/CT is usually not as helpful in primary lesion characterization. FDG avid ground glass nodules are often infectious or inflammatory, but non FDG avid ground glass nodules could still represent adenocarcinoma. In the case of part solid lesions, PET/CT may have utility for the detection of FDG uptake in the solid component.
The utility of 18F-FDG PET/CT in bronchogenic carcinoma can be separated into staging, prognosis, and post treatment imaging.
NSCLC staging
NSCLC staging with 18F-FDG PET/CT is a widely supported practice, endorsed by multiple organizations including the National Comprehensive Cancer Network (NCCN), the American College of Radiology Appropriateness Criteria, the Society of Nuclear Medicine and Molecular Imaging, and the American College of Chest Physicians (18-20). NSCLC staging is based on the TNM staging criteria. Overall, the addition of PET/CT in lung cancer staging can affect the final lung cancer stage, with studies showing the addition of PET/CT may lead to upstaging in 6.4–41.1% of patients and downstaging in 9.5–12.3% of patients (21,22).
NSCLC T staging
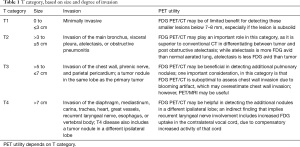
Lung cancer T staging describes the tumor size and invasive features and is separated into four categories, T1–T4 (23). Specific PET imaging characteristics are listed for each (Table 1).
Full table
NSCLC N staging
The N staging is used to classify lymph node involvement and is separated into three categories N1–N3. N1 nodes include ipsilateral intrapulmonary, peribronchial, and hilar lymph nodes. N2 nodes include ipsilateral mediastinal or subcarinal lymph nodes. N3 nodes include contralateral hilar or mediastinal lymph nodes, ipsilateral or contralateral scalene nodes, and supraclavicular lymph nodes. A positive lymph node is defined as a SUV max greater than the SUV max of the mediastinal blood pool.
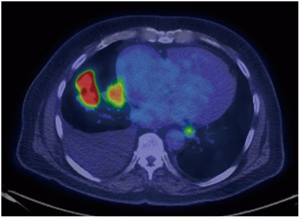
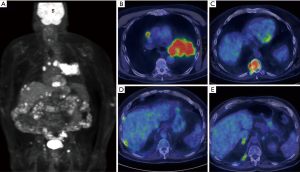
Lung cancer N staging is where the utility of PET/CT has excelled due to limitations of conventional CT imaging. While conventional imaging can accurately demonstrate lymph node size, PET/CT can also image metabolic differences within a lymph node (Figure 6). In a study by Gould et al. in 2003, nodal metastases detection by conventional CT had sensitivity of 61% and specificity of 79%. With PET/CT, this was improved to a sensitivity of 85% and a specificity of 90% (24). These results were later confirmed in a 2013 meta-analysis of 56 studies with pooled FDG PET/CT sensitivities and specificities of 72% and 91% respectfully in determining mediastinal nodal staging. The value of this improved detection has a direct impact on patient care. In the findings of a prospective multicenter trial in 2015, management strategies changed in approximately 72% of cases of lung cancer when FDG PET/CT examinations were used (25).
NSCLC M staging
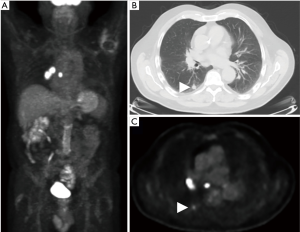
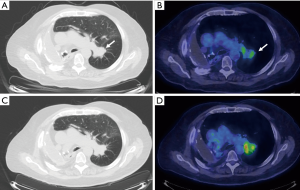
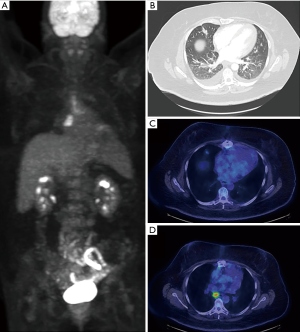
Utility of FDG-PET in lung cancer M staging is shown in Table 2. PET/CT is excellent for detecting distant metastases, including metastases in the contralateral lung (Figure 7). In a recent meta-analysis, the sensitivity, and specificity of 18FDG PET/CT for all distant metastasis was 93% and 96% respectively (26). Bone metastases were detected with a sensitivity of 90% and specificity of 98% (27). PET/CT is very helpful for adrenal metastases with a sensitivity of 93% and specificity of 90% (28). PET/CT is also excellent for liver metastases, however, this is less well studied. The major weakness of PET/CT in the setting of M disease is for the evaluation of brain metastases due to the high background normal brain activity (24). However, brain metastases can occasionally be detected (Figure 8), appearing as either as relatively hypermetabolic or hypometabolic foci.

Full table
False positive
While the diagnostic accuracy of PET is quite good, reaching up to 93.5%, this still leaves a 6.5% false positive rate (29). The most common causes of false positives are due to inflammatory psuedotumor and tuberculosis. False positives are more likely in the elderly, diabetics, and were associated with increased interleukin-6 (IL-6) levels, or positive T-spot tuberculosis tests (29).
False negative
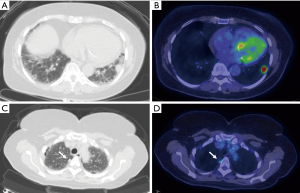
False-negative findings at PET can be the result of a small nodule size, low cellular density in lesions or low tumor avidity for FDG. Common false negative histologies included minimal to noninvasive adenocarcinomas (Figure 9), mucinous adenocarcinomas, lymphoma, and carcinoid tumor (15,30,31). The most important radiologic factor for risk assessment is lesion stability versus change over serial imaging (25). In solitary pulmonary nodules that demonstrate negative findings at PET, serial CT follow-up imaging may be performed in a patient with a low pretest likelihood of malignancy. In a patient with a high pretest likelihood of malignancy, tissue sampling or resection should be considered (32).
SCLC
SCLC has traditionally been staged via the Veterans Administration Lung Study Group (VALSG) 2-stage classification scheme: limited-stage (LS-SCLC) or Extensive-stage (ES-SCLC). Recently, the IASLC proposed that the newly revised TNM staging classification for lung cancer should replace the VALSG system for SCLC (33).
Findings at FDG PET/CT can lead to a change in initial management in up to 27% of patients with SCLC, and a change in overall disease stage in 32% of patients. In addition, the radiation field can be modified in up to 68% of patients due to overall improved characterization of intrathoracic disease (34-37).
Bronchogenic carcinoma prognosis
While PET/CT is a valuable resource in defining bronchogenic carcinoma anatomically with regards to TNM staging, it also holds important prognostic information in a variety of quantifiable PET parameters. SUV max measures only the highest SUV measurement in a single voxel within a region of interest. Studies have shown that SUV max alone does not significantly correlate with survival outcomes, particularly with SCLC (38,39). Additional parameters that can predict lung cancer prognosis include metabolic tumor volume (MTV) and total lesion glycolysis (TLG). MTV is calculated using a fixed voxel-based SUV threshold and sums all voxels with SUV values greater than or equal to the threshold. TLG is calculated by multiplying MTV by the mean SUV max. Recent studies have found that high MTV and high TLG were associated with a significantly poorer prognosis in NSLC and SCLC (40,41).
Bronchogenic carcinoma post treatment imagining
After curative treatment of bronchogenic carcinoma, surveillance imaging with CT is recommended every 6 months for 2 years and annually after 2 years by the American Society of Clinical Oncology (42). Despite evidence of high diagnostic performance with improved sensitivity and specificity over CT for recurrence, PET/CT is not recommended (43). Some of the cited reasons include increased cost and radiation exposure of PET/CT (42). However, one recognized application of PET/CT post therapy includes the differentiation of tumor progression or radiation pneumonitis. Radiation pneumonitis may potentially be differentiated from residual or recurrent tumor mainly based off of pattern or FDG uptake. The three patterns of radiation pneumonitis described by Iravani et al. include subpleaural/patchy, diffuse, and peripheral (34).
18F-FDG PET/MRI
Despite challenges in MR imaging of the chest due to low proton density and respiratory motion, 18F-FDG PET/MRI may still play a role in evaluation of a variety of thoracic disease processes (44).
Pulmonary nodules
Current 18F-FDG PET/MRI techniques are highly sensitive for the detection for FDG-avid solid pulmonary nodules measuring as small as 5 mm (45). New approaches such as ultrashort echo time sequences (UTE) have been proposed to improve detection of smaller pulmonary nodules, but detection of nodules smaller than 5mm remains challenging (46,47).
Bronchogenic carcinoma
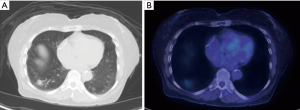
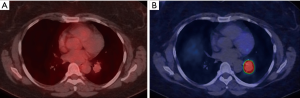
MRI provides greater tissue contrast resolution compared to CT, which may improve delineation of chest wall (Figure 10), diaphragm, or mediastinal invasion, which has implications for T-staging. 18F-FDG PET/MRI has been proven to have equivalently high diagnostic performance for T and N staging of NSCLC compared to 18F-FDG PET/CT (35). 18F-FDG PET/MRI may have greater sensitivity in detecting mediastinal lymph nodes, however, the clinical impact of different staging results has not been fully investigated (36). In terms of M-staging, MRI offers excellent characterization of common metastatic locations such as the adrenal glands, liver, and the brain, which is already a requirement for staging of advanced lung cancer. In this manner, exams can be tailored to combine the 18F-FDG PET and MRI information in one examination, providing convenience for patients and referring providers.
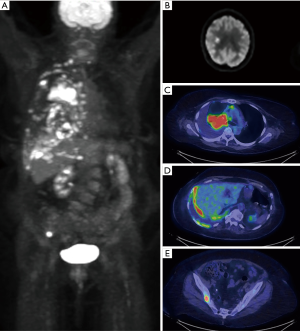
Neuroendocrine malignancy
Major strides have been made in the imaging of neuroendocrine tumors including bronchial carcinoid due to the development of PET radiotracers targeting somatostatin receptors. While bronchial carcinoid tumors typically have mild to moderate FDG uptake, the density of somatostatin receptors allows for high quality imaging using 68Ga DOTATATE as demonstrated in Figure 11 (14). Typically, carcinoid tumors will show low 18F-FDG avidity and high 68Ga DOTATATE avidity, however this is dependent on the degree of differentiation with more poorly differentiated tumors having greater FDG uptake and lower DOTATATE avidity (37).
68Ga DOTATATE has been proven to be superior to previous forms of somatostatin receptor imagining, such as 111In-pentetreotide (48). Meta-analysis of the sensitivity and specificity of 68Ga DOTATE for detecting neuroendocrine tumors including pulmonary carcinoid tumors measures 93% and 91% respectively (49). The utility and superior imagining characteristics of 68Ga DOTATATE have led to change in management for up to 36% of patients (48).
One of the most exciting possibilities for neuroendocrine tumors is the role of peptide receptor radionuclide therapy (PRRT) with 177Lu DOTATATE. 177Lu DOTATATE contains the same somatostatin receptor analog, DOTATATE, however instead of the 68Ga radionuclide used for diagnostic imaging, 177Lu is incorporated. The therapeutic agent binds to the cell surface somatostatin receptor and then undergoes β- radioactive decay, damaging DNA and resulting in cellular apoptosis. PRRT with 177Lu DOTATATE for lung carcinoid tumor was not studied under the NETTER-1 trial of gastrointestinal neuroendocrine tumors, however, preliminary evidence suggests similar efficacy and safety of PRRT in patients with pulmonary carcinoid tumor (50,51).
Conclusions
Capitalizing on the strength of combining anatomic and physiological imaging, nuclear medicine, and specifically PET, serves a vital role in the evaluation of thoracic disorders, enhancing the care of patients. This invaluable clinical tool includes well-recognized applications such as in evaluation of pulmonary nodules and staging of bronchogenic carcinoma. Emerging technologies have found new roles in PET/MRI and 68Ga DOTATATE PET, as well as radionuclide therapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Chi Wan Koo) for the series “Contemporary Practice in Thoracic Neoplasm Diagnosis, Evaluation and Treatment” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/jtd-2019-cptn-09). The series “Contemporary Practice in Thoracic Neoplasm Diagnosis, Evaluation and Treatment” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lillington GA. Management of solitary pulmonary nodules. Disease-a-Month 1991;37:269-318. [Crossref] [PubMed]
- Bunyaviroch T, Coleman RE. PET evaluation of lung cancer. J Nucl Med 2006;47:451-69. [PubMed]
- Bury T, Dowlati A, Paulus P, et al. Evaluation of the solitary pulmonary nodule by positron emission tomography imaging. Eur Respir J 1996;9:410-4. [Crossref] [PubMed]
- Higashi K, Matsunari I, Ueda Y, et al. Value of whole-body FDG PET in management of lung cancer. Ann Nucl Med 2003;17:1-14. [Crossref] [PubMed]
- Hochhegger B, Alves GR, Irion KL, et al. PET/CT imaging in lung cancer: indications and findings. J Bras Pneumol 2015;41:264-74. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Christensen JA, Nathan MA, Mullan BP, et al. Characterization of the solitary pulmonary nodule: 18F-FDG PET versus nodule-enhancement CT. AJR Am J Roentgenol 2006;187:1361-7. [Crossref] [PubMed]
- Behzadi A, Ung Y, Lowe V, et al. The role of positron emission tomography in the management of non-small cell lung cancer. Can J Surg 2009;52:235-42. [PubMed]
- Lowe VJ, Hoffman JM, DeLong DM, et al. Semiquantitative and visual analysis of FDG-PET images in pulmonary abnormalities. J Nucl Med 1994;35:1771-6. [PubMed]
- Cronin P, Dwamena BA, Kelly AM, et al. Solitary pulmonary nodules: meta-analytic comparison of cross-sectional imaging modalities for diagnosis of malignancy. Radiology 2008;246:772-82. [Crossref] [PubMed]
- Daniels CE, Lowe VJ, Aubry MC, et al. The utility of fluorodeoxyglucose positron emission tomography in the evaluation of carcinoid tumors presenting as pulmonary nodules. Chest 2007;131:255-60. [Crossref] [PubMed]
- Nagelschneider AA, Broski SM, Holland WP, et al. The flip-flop fungus sign: an FDG PET/CT sign of benignity. Am J Nucl Med Mol Imaging 2017;7:212-7. [PubMed]
- Johnson GB, Peller PJ, Kemp BJ, et al. Future of thoracic PET scanning. Chest 2015;147:25-30. [Crossref] [PubMed]
- Erasmus JJ, McAdams HP, Patz EF Jr, et al. Evaluation of primary pulmonary carcinoid tumors using FDG PET. AJR Am J Roentgenol 1998;170:1369-73. [Crossref] [PubMed]
- Berger KL, Nicholson SA, Dehdashti F, et al. FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am J Roentgenol 2000;174:1005-8. [Crossref] [PubMed]
- Erasmus JJ, Macapinlac HA. Low-sensitivity FDG-PET studies: less common lung neoplasms. Semin Nucl Med 2012;42:255-60. [Crossref] [PubMed]
- Chun EJ, Lee HJ, Kang WJ, et al. Differentiation between malignancy and inflammation in pulmonary ground-glass nodules: The feasibility of integrated 18F-FDG PET/CT. Lung Cancer 2009;65:180-6. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-e50S.
- Ravenel JG, Rosenzweig KE, Kirsch J, et al. ACR Appropriateness Criteria non-invasive clinical staging of bronchogenic carcinoma. J Am Coll Radiol 2014;11:849-56. [Crossref] [PubMed]
- Takeuchi S, Khiewvan B, Fox PS, et al. Impact of initial PET/CT staging in terms of clinical stage, management plan, and prognosis in 592 patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 2014;41:906-14. [Crossref] [PubMed]
- Gregory DL, Hicks RJ, Hogg A, et al. Effect of PET/CT on management of patients with non-small cell lung cancer: results of a prospective study with 5-year survival data. J Nucl Med 2012;53:1007-15. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med 2003;139:879-92. [Crossref] [PubMed]
- Kubota K, Matsuno S, Morioka N, et al. Impact of FDG-PET findings on decisions regarding patient management strategies: a multicenter trial in patients with lung cancer and other types of cancer. Ann Nucl Med 2015;29:431-41. [Crossref] [PubMed]
- Li J, Xu W, Kong F, et al. Meta-analysis: accuracy of 18FDG PET-CT for distant metastasis staging in lung cancer patients. Surg Oncol 2013;22:151-5. [Crossref] [PubMed]
- Bury T, Barreto A, Daenen F, et al. Fluorine-18 deoxyglucose positron emission tomography for the detection of bone metastases in patients with non-small cell lung cancer. Eur J Nucl Med 1998;25:1244-7. [Crossref] [PubMed]
- Kumar R, Xiu Y, Yu JQ, et al. 18F-FDG PET in evaluation of adrenal lesions in patients with lung cancer. J Nucl Med 2004;45:2058-62. [PubMed]
- Feng M, Yang X, Ma Q, et al. Retrospective analysis for the false positive diagnosis of PET-CT scan in lung cancer patients. Medicine 2017;96:e7415. [Crossref] [PubMed]
- Kuriyama K, Seto M, Kasugai T, et al. Ground-glass opacity on thin-section CT: value in differentiating subtypes of adenocarcinoma of the lung. AJR Am J Roentgenol 1999;173:465-9. [Crossref] [PubMed]
- Koss MN, Hochholzer L, Nichols PW, et al. Primary non-Hodgkin's lymphoma and pseudolymphoma of lung: a study of 161 patients. Hum Pathol 1983;14:1024-38. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-e120S.
- Kalemkerian GP, Gadgeel SM. Modern staging of small cell lung cancer. J Natl Compr Canc Netw 2013;11:99-104. [Crossref] [PubMed]
- Iravani A, Turgeon G-A, Akhurst T, et al. PET-detected pneumonitis following curative-intent chemoradiation in non-small cell lung cancer (NSCLC): recognizing patterns and assessing the impact on the predictive ability of FDG-PET/CT response assessment. Eur J Nucl Med Mol Imaging 2019;46:1869-77. [Crossref] [PubMed]
- Kirchner J, Sawicki LM, Nensa F, et al. Prospective comparison of (18)F-FDG PET/MRI and (18)F-FDG PET/CT for thoracic staging of non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2019;46:437-45. [Crossref] [PubMed]
- Schaarschmidt BM, Grueneisen J, Metzenmacher M, et al. Thoracic staging with (18)F-FDG PET/MR in non-small cell lung cancer - does it change therapeutic decisions in comparison to (18)F-FDG PET/CT? Eur Radiol 2017;27:681-8. [Crossref] [PubMed]
- Kayani I, Bomanji JB, Groves A, et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1,Tyr3-octreotate) and 18F-FDG. Cancer 2008;112:2447-55. [Crossref] [PubMed]
- Cuccurullo V, Mansi L. AJCC cancer staging handbook: From the AJCC cancer staging manual. Springer, 2011.
- Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 1.2015. J Natl Compr Canc Netw 2014;12:1738-61. [Crossref] [PubMed]
- Chen HH, Chiu NT, Su WC, et al. Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non–small cell lung cancer. Radiology 2012;264:559-66. [Crossref] [PubMed]
- Liao S, Penney BC, Wroblewski K, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2012;39:27-38. [Crossref] [PubMed]
- Schneider BJ, Ismaila N, Aerts J, et al. Lung Cancer Surveillance After Definitive Curative-Intent Therapy: ASCO Guideline. J Clin Oncol 2020;38:753-66. [Crossref] [PubMed]
- Antoniou AJ, Marcus C, Tahari AK, et al. Follow-up or Surveillance (18)F-FDG PET/CT and Survival Outcome in Lung Cancer Patients. J Nucl Med 2014;55:1062-8. [Crossref] [PubMed]
- Ehman EC, Johnson GB, Villanueva-Meyer JE, et al. PET/MRI: Where might it replace PET/CT? J Magn Reson Imaging 2017;46:1247-62. [Crossref] [PubMed]
- Chandarana H, Heacock L, Rakheja R, et al. Pulmonary nodules in patients with primary malignancy: comparison of hybrid PET/MR and PET/CT imaging. Radiology 2013;268:874-81. [Crossref] [PubMed]
- Burris NS, Johnson KM, Larson PE, et al. Detection of Small Pulmonary Nodules with Ultrashort Echo Time Sequences in Oncology Patients by Using a PET/MR System. Radiology 2016;278:239-46. [Crossref] [PubMed]
- Crimi F, Varotto A, Orsatti G, et al. Lung visualisation on PET/MRI: implementing a protocol with a short echo-time and low flip-angle volumetric interpolated breath-hold examination sequence. Clin Radiol 2020;75:239.e15-e21. [Crossref] [PubMed]
- Deppen SA, Liu E, Blume JD, et al. Safety and efficacy of 68Ga-DOTATATE PET/CT for diagnosis, staging, and treatment management of neuroendocrine tumors. J Nucl Med 2016;57:708-14. [Crossref] [PubMed]
- Treglia G, Castaldi P, Rindi G, et al. Diagnostic performance of Gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastroenteropancreatic neuroendocrine tumours: a meta-analysis. Springer, 2012.
- Strosberg J, Wolin E, Chasen B, et al. 6LBA 177-Lu-Dotatate significantly improves progression-free survival in patients with midgut neuroendocrine tumours: results of the phase III NETTER-1 trial. Eur J Cancer 2015;51:S710. [Crossref]
- Naraev BG, Ramirez RA, Kendi AT, et al. Peptide Receptor Radionuclide Therapy for Patients With Advanced Lung Carcinoids. Clin Lung Cancer 2019;20:e376-92. [Crossref] [PubMed]


