Therapy in stable chronic obstructive pulmonary disease patients with pulmonary hypertension: a systematic review and meta-analysis
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by dyspnea and exercise limitation, most commonly attributed to airflow obstruction, hyperinflation, gas exchange abnormalities, and inspiratory muscle weakness (1). The 5th world symposium on pulmonary arterial hypertension (PAH) (Nice France) classified “Pulmonary hypertension (PH) associated with lung diseases and/or hypoxemia” into group three (2). Although modest in severity, PH is present at exercise in many individuals with COPD (3). Several studies in Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage IV patients revealed that mean pulmonary artery pressure (mPAP) of more than 90% patients was >20 mmHg, with most ranging between 20 and 35 mmHg and about 3% to 5% patients with mPAP >35 to 40 mmHg (4,5). COPD complicated PH would make adverse effects on survival and exercise capacity, and PH is a vital risk factor of acute exacerbations. Lots of clinical trials have proved that, the application of PH specific drugs in PAH (mainly group 1) could play pulmonary vasodilation and anti-endothelial proliferation effects, thereby reducing pulmonary vascular resistance (PVR) and right ventricle (RV) afterload (6). Nevertheless, in addition to oxygen therapy in hypoxemia, whether PH specific therapies could be used in COPD associated PH is uncertain. Current guidelines recommend that, PH specific therapies should be taken into account when PH continues in spite of optimizing COPD management, and when the degree of lung parenchymal abnormalities is out of proportion to the severity of PH. Theoretically, PH-specific therapy could reduce pulmonary artery pressure, improve RV function (7), and enhance exercise endurance for COPD patients (8). Unfortunately, there were only some case reports or a few of randomized clinical trials. It is still unclear that whether COPD patients could really get benefit from PH specific therapy or not. With that, a systematic review and meta-analysis was performed to estimate whether PH-specific therapies could enhance exercise capacity, reduce PAP and improve life quality in COPD associated PH patients.
Materials and methods
Search strategy
Publications applying PH-specific therapy in COPD patients were searched by PubMed (up to 28th February 2014), Cochrane Register of Controlled Trials (CENTRAL) of the Cochrane online (2014), China Knowledge Resource Integrated Database (CNKI). The key terms were: “COPD” or “chronic bronchitis” or “emphysema” and “vasodilator agent” or “phosphodiesterase 5 inhibitor” or “endothelin receptor antagonist” or “rho-kinase inhibitor” or “prostacyclin” or “prostacyclin derivative” or “sildenafil” or “iloprost” or “tadalafil” or “udenafil” or “riociguat” or “epoprostenol” or “treprostinil” or “beraprost” “bosentan” or “ambrisentan” or “sitaxentan”. The bibliographies of relevant review articles were also identified. Moreover, we investigated those ongoing or just completed registration trials on “ClinicalTrials.gov”. Searching was no restricted into specific language and released time was until February 28, 2014.
Inclusion and exclusion criteria
Randomized controlled trials (RCTs) were included, in which the diagnosis of all subjects conformed to the definition of COPD and the standardization of PH according to current guidelines as the author described. Other lung diseases (such as interstitial lung disease) and other classifications of PH (group1, 2, 4 and 5) were excluded. The trial time was more than 4 weeks. Exercise capacity was the major outcome measured by 6-minute walking test (6MWT) or endurance time in constant exercise test (CWET). Secondary outcome were PAP, arterial partial pressure of oxygen, health-related quality-of-life and so on. We analyzed each trial according to prospective randomized controlled design. Researches on animal or cell, case reports, and the language except for English or Chinese were excluded. Two authors independently assessed all studies to determine whether they met the inclusion criteria, and if there was a disagreement, they negotiated with the third author.
Data collection and analysis
Data of the study applying PH-specific therapy in COPD patients were extracted. The variables of each study were summarized including authors, publication journal, published year and country, number and disease diagnosis of study population, usage and dosage of PH specific drug, and period of treating time. We got data from formal published articles except one conference abstract. The potency assay of exercise capacity reflected by 6-min walking distance (6MWD) was the main outcome. We assessed the methodological quality of each trial as Cochrane Handbook of Systematic Reviews recommended. Risk of bias that may affect the cumulative evidence was evaluated by random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other potential bias.
Review Manager version 5.3 (RevMan; The Cochrane Collaboration, Oxford, UK) was used to conduct meta-analysis. We followed the criteria of Cochrane Handbook to assess the methodological heterogeneity (9). In Cochrane’s Q statistic, P<0.1 indicated significant difference. The I2 statistic described the percentage of variation between trials, ranging from 0-100%, which determined whether fixed-effect model or random-effect model was appropriate for analyzing. The value of 0% indicated no heterogeneity, and critical value of 25%, 50%, 75% were taken as low, moderate and high heterogeneity respectively. Fixed-effect model was chosen according to pre-analyzed inherent differences and heterogeneity. The mean difference (MD) and 95% confidence intervals (CI) for indexes of 6MWD, PAP, and so on were calculated in COPD patients after PH specific drug treating.
Results
Characteristics of included articles
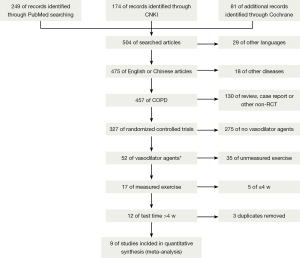
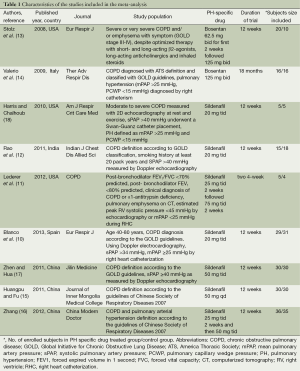
We searched 504 literatures, of which 495 were excluded because of various reasons according to the inclusion criteria (Figure 1 showed the detail of process), the other nine published between 2008 and 2013, and among them, eight full texts were available and one was conference abstract (10-18). Table 1 listed the characteristics of nine trials which were all randomized, double-blind, placebo-controlled, parallel group design. A total of 365 subjects that were diagnosed as COPD according to GOLD guideline (post-bronchodilator FEV1/FVC <70% in pulmonary function test, FEV1: forced expired volume in 1 second, FVC: forced vital capacity) were involved. We accepted the definition of PH the author applied by right heart catheterization (mean PAP ≥25 mmHg) or echocardiography (systolic PAP >30 mmHg). Given that subjects of seven trials were diagnosed as PH, those of two trials were without PH at rest. Subjects were treated with sildenafil in seven studies, while the other two with bosentan. The duration of treatment time ranged from 4 weeks to 18 months and mostly it was 12 weeks. To compare the effect of PH specific therapy, all trials tested 6MWD, while 3 trials performed CWET; however maximal oxygen uptake (VO2 max) was measured by different units (mL/min/kg and mL/min, respectively) that were not suitable for cumulative statistics. Four trials measured arterial partial pressure of oxygen (PaO2) as the second outcome to appraise the improvement of hypoxia. To observe whether PH specific drug could reduce PAP, six trials compared the change of PAP from the baseline. Regarding the health-related quality-of-life questionnaires, two trials investigated St. George’s Respiratory Questionnaire (SGRQ), and another two evaluated Short Form 36 health survey (SF-36). As to whether PH-specific treatment could improve pulmonary function, seven trials mentioned FEV1 with different methods (FEV1 liters, FEV1 liters/second, FEV1% predicted, and FEV1% post-bronchodilator, respectively), and there were no significant differences (data not shown).
Full table
Risk of bias assessment
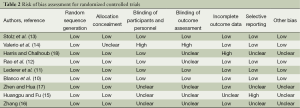
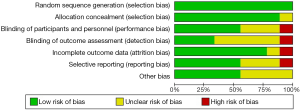
The quality of included studies was assessed for risk of bias by Revman as described in Cochrane Handbook. The assessment for RCTs consisted of sequence generation, allocation concealment, blinding, incomplete outcomes, selective reporting and other unknown bias. The judgment to the performance of RCTs was “low” or “unclear” or “high” risk of bias. These were showed in Table 2 and Figure 2. Each trial informed dropout number, however there was no obvious difference in the overall withdrawal rate between PH specific drug therapy group and control group (5.5% vs. 2.9%, P=0.45).
Full table
Effects of PH specific therapy
All nine trials determined 6MWD as main outcome to evaluate the effects of COPD patients treated with PH specific drug. Analysis in Figure 3 revealed that there was an outstanding heterogeneity among nine trials (I2=65%, P<0.00001). Pooled analysis indicated that, 6MWD in COPD patients increased by 66.39 m (95% CI: 59.44-73.34), demonstrating that PH specific therapy could improve exercise capacity. Based on diagnosis of PH by RHC or echocardiography, nine trials were divided into two subgroups. Subgroup analysis indicated that 6MWD in patients that were confirmed PH increased (MD 67.24 m, 95% CI: 60.26-74.23), while those without PH at rest did not (MD −9.24 m, 95% CI: −75.08 to 56.61), which meant PH specific drug might be effective only to COPD patients with definite PH diagnosis and without preventive effect for susceptible population of PH. As to particular drug, there was a trend for bosentan to improve exercise capacity, while treated with sildenafil increased 6MWD by 66.84 m from baseline (95% CI: 59.85-73.82). There was significant difference according to the drug (P<0.00001 shown in Figure 4).
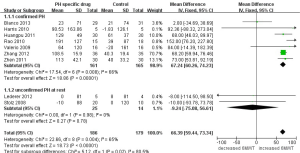

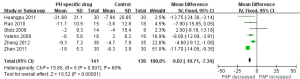
As previous trials demonstrated PH specific drugs, such as bosentan and sildenafil could obviously decrease PAP to other kinds of PH, especially idiopathic pulmonary arterial hypertension (IPAH). We investigated six trials. As the result shown in Figure 5, PH specific drug could also lower PAP 9.02 mmHg (95% CI: −10. 71 to −7.34).
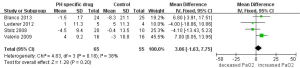
Once some research pointed out the reason why PH specific drugs could not be used in COPD patients was their feature of pulmonary vascular dilatation, which would deteriorate hypoxemia due to the imbalance of ventilation/perfusion (V/Q) ratio. Thus this review analyzed whether it was true or not. PaO2 is a main index to monitor hypoxemia. Four trials measured PaO2 (Figure 6). It appeared that PaO2 could be increased slightly after PH specific therapy (MD 3.06 mmHg, 95% CI: −1.63 to 7.75). Since there was no obvious improvement for hypoxemia, how was the symptom of dyspnea in COPD patients? We tried to perform a meta-analysis for dyspnea. Five trials reported dyspnea index with different scoring systems, of which two only reported the baseline and no difference in conclusion. We selected two trials using Borg dyspnea index to analyze. Figure 7 showed that the symptom of dyspnea seemed to be improved (MD −0.86, 95% CI: −1.86 to 0.14).
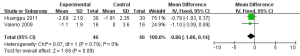
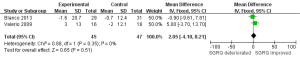
Although hypoxemia had not been recovered strikingly, we wondered whether health relatedly life quality could be improved. There were four trials mentioned SF-36, and we could only get integrated results from one. Two trials evaluated SGRQ. It appeared that PH specific drug can improve SGRQ, but without notable difference (Figure 8, MD 2.05, 95% CI: −4.10 to 8.21). However, as the number of trials and participants conducted in dyspnea index and SGRQ were too little, it could not be final concluded.
Discussion
PH is a common complication of COPD without effective drugs to treat (19). Lots of clinical trials have proved the effect of new drugs for PAH (20). These PH specific drugs stimulated interest of their potential application value in other kinds of PH; such as COPD associated PH. Reports on the effect of PH specific drugs on exercise capacity in COPD are somewhat conflicting. Some studies have found that PH is a limiting factor of the performance in exercise tests such as the 6MWT and the cardiopulmonary exercise test (CPET), while another study showed no differences in 6MWT between COPD patients with or without moderate PH defined by an mPAP >30 mmHg (21-23). Our systematic review and meta-analysis indicated that exercise capacity of COPD patients that were confirmed severe PH (mPAP >35 mmHg by RHC or sPAP >50 mmHg by echocardiography) could be statistically improved with PH specific therapy. In addition to the effect on PAP, treatment might have an ameliorated effect on dyspnea.
Hypoxic pulmonary vasoconstriction is considered a major driver of the development of PH in COPD and interstitial lung disease (24). Similar to IPAH, pathology of pulmonary arteries in COPD patients exhibited fibromuscular intimal thickening and proliferation in smooth muscle cells and endothelial cells (25). So the question whether pulmonary vasodilators used in PAH is useful for lung disease-related PH comes forward. Mainly three kinds of specific pulmonary vasodilators are used in clinical practice to treat PAH, namely phosphodiesterase-5 (PDE-5) inhibitors, endothelin receptor antagonists (ERAs) or prostacyclin analogs. Sildenafil is a PDE-5 inhibitor, inhibiting the breakdown of cyclic guanylyl monophosphate (cGMP) which is the second messenger downstream to NO (nitric oxide). It has been proved that sildenafil could improve exercise tolerance in PAH (26).
Endothelin-1 (ET-1) exerts vasoconstriction and stimulates cell proliferation through activation of the ET A receptor which is localized in the vascular smooth muscle cells. ET-1 also binds to the ET B receptor which is situated in vascular smooth muscle where it evokes vasoconstriction, but the ET B receptor is also situated in vascular endothelial cells, where it stimulates release of NO and prostacyclin (27). Bosentan is a non-selective ET-1 receptor antagonist. However, a beneficial effect of bosentan treatment in patients with COPD and PH has been less observed, and two studies we selected contradict each other.
Epoprostenol, treprostinil and iloprost are prostacyclin analogs used for PAH (28). Epoprostenol has a very short half-life and is administered by continuous intravenous infusion. Treprostinil has the longest half-life of the parenteral formulations. Both treprostinil and iloprost can be administered by intravenous or subcutaneous injection as well as by inhalation. Given the property, the prostacyclins could cause hypotension and holds the risk of vasodilation in poorly ventilated areas with a resultant intrapulmonary shunt and hypoxemia. Moreover, COPD is a chronic progress, intravenous or subcutaneous injection may be not suit for permanently therapy. Intravenous infusion of prostacyclin has been compared to inhaled prostacyclin in patients with pulmonary fibrosis (29). Nonetheless, there was only one study to COPD related PH, which was not blinded or placebo controlled (30).
As to these conditions, our meta-analysis selected studies using sildenafil or bosentan, and mostly were about sildenafil, prostacyclin analogs were not included.
It was previously considered that PH specific drug treating failure in COPD patients was due to deterioration of imbalance of V/Q ratio and hypoxemia (31,32). Specifically, the ventilation function of alveolar units in COPD was poor, vasodilators dilated pulmonary arteries yet did not correct hypoxia, which would worsen V/Q disproportion (33). However, our analysis demonstrated that PH specific drug seemed to increase PaO2, even a little, rather than deteriorate oxygenation. The implication was that so-called adverse effect of PH specific drug on V/Q ratio of COPD patients might be very little. Recently a study in COPD patients has shown that out patients with severe PH (mPAP >35 mmHg) had an evidently lower 6MWT compared to those without PH after correction for lung function, age, sex and BMI, where as those with mild PH (25 mmHg < mPAP <35 mmHg) did not have a significantly shorter 6MWT (34). It indicates that only severe PH seems to restrict exercise capacity per se by impairing cardiac function. On the other hand, in patients with mild PH with symptoms caused by impaired ventilation, pulmonary vasodilation may not relieve symptoms, but aggravate V/Q mismatch. All included studies in our meta-analysis measured PAP, of which mPAP was above 35 mmHg or sPAP >50 mmHg except for Stolz and Harris. This speaks in favor of specific treatment of PH with off-loading of the RV in patients with severe PH. It also supports the thesis that PH specific therapy could reduce afterload of RV in COPD patients with serve PH.
According to current guidelines of PH, PH specific drugs could be used in out of proportion PH due to lung disease. However, existing RCTs studies did not include this part of population, which limited their application (35). Present experience of PH specific drugs for COPD associated PH from published articles were little, including only a small amount of studies evaluating short-term effect, or some uncontrolled studies and case reports. COPD is a chronic disease process, while needing long treatment and getting persistent curative effect. Given this, our analysis selected trials that were more than 4 weeks. On the other hand, PH is an important risk factor for COPD exacerbation, meaning more frequent during the course of disease, yet the duration of RCTs is usually long. This may explain the reason why the number of RCTs on the application of PH specific therapy in COPD patients is so little.
Our analysis was hampered by heterogeneity. Principally, those papers with all languages should be included for meta-analysis, however, as to language limitation, we only included English or Chinese, which might cause selection bias. As primary outcome, the statistics are integrated, but to secondary outcome, some only presented baseline or final value, which could not be converted into the change value. And not all studies measured arterial oxygen partial pressure, Borg dyspnea index and health related life, making us unable to evaluate the real benefit of PH-specific treatment. The meta-analysis of these outcomes cannot be final conclusion, it need more data in the future. Traditional treatment for COPD generally could improve pulmonary function, while these PH-specific drugs seem not. The reason was too little subjects of trials included or short treating time was unknown. The rate of decline in FEV1 of GOLD stage III-IV COPD patients was less than that in GOLD stage II (36), conversely the rate of recover might be the same. Most of PH in COPD patients in our review were severe, it could be speculated that the data of pulmonary function recovered might be too small for statistics. Pulmonary function was conducted as a main outcome in clinical trials with COPD patients, while main outcome in PAH patients with PH specific therapy was exercise capacity that was also applied in some studies of COPD associated PH. In the view of the basis of disease, it should be comprehensively access the benefit from PH specific therapy, such as improvement of pulmonary function, exercise capacity, health-related life-of-quality, not just only exercise capacity or pulmonary artery pressure. Perhaps the improvement of exercise capacity was not obvious, if not worse, pulmonary function was significantly improved, it still could be said that COPD patients could benefit from that treatment. Hypoxia is the primary cause of PH in COPD, so long term oxygen treatment is most recommended (37). Moreover, an optimal bronchodilator therapy is needed. Not all studies clarified if the bronchodilator had been used, nor the brand had been pointed out. It could not eliminate the effect of bronchodilators to PH completely. There were many kinds of methods for drug clinical trial research, as for meta-analysis, we only selected RCTs. The results of RCTs may not suit for all COPD patients or truly reflect the effectiveness from the perspective of patients. Further analysis should include observational study or real word trials (RWTs) together to compensate the defect.
Conclusions
PH specific drugs could improve exercise capacity and reduce pulmonary artery pressure of COPD patients with severe PH. Nevertheless the benefit of PH specific treatment was not obvious to those without PH at rest that might be potential susceptible populations to PH. Despite of mild improvement of dyspnea, there was no significant elevated oxygen partial pressure and improved health related life quality associated with PH-specific treatment. According to this systematic review and meta-analysis, we propose that PH specific drugs (especially sildenafil) could be used in COPD patients with severe PH. Future studies should enroll more subjects to determine which is the real indicator for the benefit of COPD associated PH from PH specific therapy (exercise capacity or pulmonary function or some else), how to carry out the risk-benefit balance.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- O’Donnell DE, Banzett RB, Carrieri-Kohlman V, et al. Pathophysiology of dyspnea in chronic obstructive pulmonary disease: a roundtable. Proc Am Thorac Soc 2007;4:145-68. [PubMed]
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34-41. [PubMed]
- Kessler R, Faller M, Weitzenblum E, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med 2001;164:219-24. [PubMed]
- Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;172:189-94. [PubMed]
- Andersen KH, Iversen M, Kjaergaard J, et al. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant 2012;31:373-80. [PubMed]
- Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173:1023-30. [PubMed]
- Chin KM, Kim NH, Rubin LJ. The right ventricle in pulmonary hypertension. Coron Artery Dis 2005;16:13-8. [PubMed]
- Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet 2001;358:1119-23. [PubMed]
- Higgins JP, Green S. eds. Cochrane handbook for systematic reviews of interventions. England; John Wiley & Sons, 2011.
- Blanco I, Santos S, Gea J, et al. Sildenafil to improve respiratory rehabilitation outcomes in COPD: a controlled trial. Eur Respir J 2013;42:982-92. [PubMed]
- Lederer DJ, Bartels MN, Schluger NW, et al. Sildenafil for chronic obstructive pulmonary disease: a randomized crossover trial. Copd 2012;9:268-75. [PubMed]
- Rao RS, Singh S, Sharma BB, et al. Sildenafil improves six-minute walk distance in chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Indian J Chest Dis Allied Sci 2011;53:81-5. [PubMed]
- Stolz D, Rasch H, Linka A, et al. A randomised, controlled trial of bosentan in severe COPD. Eur Respir J 2008;32:619-28. [PubMed]
- Valerio G, Bracciale P, Grazia D’Agostino A. Effect of bosentan upon pulmonary hypertension in chronic obstructive pulmonary disease. Ther Adv Respir Dis 2009;3:15-21. [PubMed]
- Huangpu W, Fu X. The curative effect of sildenafil on chronic obstructive pulmonary disease associated pulmonary hypertension. Journal of Inner Mogolia Medical College 2011;33:60-2.
- Zhang P. Clinical curative effect of sildenafil on pulmonary hypertension. China Modern Doctor 2012;50:83-4.
- Zhen J, Hua S. Clinical analysis of the treatment of sildenafil in 30 chronic obstructive pulmonary disease patients combined with pulmonary hypertension. Jilin Medicine 2011;32:75-7.
- Harris KN, Chalhoub M. The effects of sildenafil in pulmonary hypertension secondary to chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;181:A5256.
- Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest 2005;127:1531-6. [PubMed]
- Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 2004;351:1425-36. [PubMed]
- Sims MW, Margolis DJ, Localio AR, et al. Impact of pulmonary artery pressure on exercise function in severe COPD. Chest 2009;136:412-9. [PubMed]
- Vonbank K, Funk GC, Marzluf B, et al. Abnormal pulmonary arterial pressure limits exercise capacity in patients with COPD. Wien Klin Wochenschr 2008;120:749-55. [PubMed]
- Pynnaert C, Lamotte M, Naeije R. Aerobic exercise capacity in COPD patients with and without pulmonary hypertension. Respir Med 2010;104:121-6. [PubMed]
- Weitzenblum E, Chaouat A, Canuet M, et al. Pulmonary hypertension in chronic obstructive pulmonary disease and interstitial lung diseases. Semin Respir Crit Care Med 2009;30:458-70. [PubMed]
- Yamakami T, Taguchi O, Gabazza EC, et al. Arterial endothelin-1 level in pulmonary emphysema and interstitial lung disease. Relation with pulmonary hypertension during exercise. Eur Respir J 1997;10:2055-60. [PubMed]
- Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005;353:2148-57. [PubMed]
- Vignon-Zellweger N, Heiden S, Miyauchi T, et al. Endothelin and endothelin receptors in the renal and cardiovascular systems. Life Sci 2012;91:490-500. [PubMed]
- Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30:2493-537. [PubMed]
- Olschewski H, Ghofrani HA, Walmrath D, et al. Inhaled prostacyclin and iloprost in severe pulmonary hypertension secondary to lung fibrosis. Am J Respir Crit Care Med 1999;160:600-7. [PubMed]
- Dernaika TA, Beavin M, Kinasewitz GT. Iloprost improves gas exchange and exercise tolerance in patients with pulmonary hypertension and chronic obstructive pulmonary disease. Respiration 2010;79:377-82. [PubMed]
- Barberà JA, Roger N, Roca J, et al. Worsening of pulmonary gas exchange with nitric oxide inhalation in chronic obstructive pulmonary disease. Lancet 1996;347:436-40. [PubMed]
- Rubin LJ. Primary pulmonary hypertension. N Engl J Med 1997;336:111-7. [PubMed]
- Blanco I, Gimeno E, Munoz PA, et al. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med 2010;181:270-8. [PubMed]
- Boerrigter BG, Bogaard HJ, Trip P, et al. Ventilatory and cardiocirculatory exercise profiles in COPD: the role of pulmonary hypertension. Chest 2012;142:1166-74. [PubMed]
- Minai OA, Chaouat A, Adnot S. Pulmonary hypertension in COPD: epidemiology, significance, and management: pulmonary vascular disease: the global perspective. Chest 2010;137:39S-51S. [PubMed]
- Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis 2012;7:95-9. [PubMed]
- Shujaat A, Bajwa AA, Cury JD. Pulmonary Hypertension Secondary to COPD. Pulm Med 2012;2012:203952.


