
Combining node location and node ratio as a prognostic factor for surgical resected non-small cell lung cancer: a population-based study
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death worldwide (1). For NSCLC patients who received radical surgical resection (lobectomy/bilobectomy/pneumonectomy and LN dissection), the status of lymph node (LN) has always been an important prognostic factor (2). The tumor-node-metastasis (TNM) staging is a widely used classification system to predict the outcome of NSCLC. But in the TNM system only the anatomic location of metastatic LN is used to define the node status (3).
Like in other solid organ malignant disease such as esophagus and breast, the number of metastatic LN has been proposed as a prognostic factor for NSCLC (4-6). However, the number of metastatic LN is confounded by the number of resected LN. In cases where few nodes were removed, the number of metastatic LN could not be accurately classified. To improve the prognostic system, the ratio between the metastatic LN and the resected LN (the LN ratio, NR), which takes into account not only the number of positive LN but also the number of LN harvested, removed the variability in nodal assessment to some extent. In recent years, there is increasing evidence indicating the NR to be significant prognostic factors for NSCLC (7-10). However, either the TNM system or the NR considers only one aspect of the LN status, which means the anatomic location or the relative number of metastatic LN. Few studies have investigated the combined effect of these two elements.
The Surveillance, Epidemiology and End Results (SEER) database is a national registry that collects cancer incidence and survival data and is representative of the US population (11). In this study, we used the SEER database to explore the prognostic value of the combined pN stage and NR (pN-NR) for NSCLC. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-758).
Methods
Data resource and study population
Approval for this study and informed consent were waived from the ethics committee in the China-Japan Friendship Hospital due to the retrospective and public database nature of this study. Patients aged between 20 and 79 years old with pN1 or pN2 NSCLC who underwent radical resection (including lobectomy, bilobectomy, pneumonectomy and LN dissection) between 2004 and 2012 were retrieved from the SEER database using the SEER-Stat V8.3.5 in February 2019. The year 2004 was chosen because the 6th edition of the TNM classification was not uniformly available in the database until then, and by the time of our study the most recently released data were in 2012. Patients who had small cell carcinoma, prior history of malignant tumor, pathological T0 or Tis disease, and stage IV disease were excluded from this study. Patients were also excluded whose number of examined LN was less than 6, who died within 30 days after surgery or without necessary clinicopathological information.
Patient records including age, gender, race, laterality, type of surgery, histological type, pathological stage, LN status, and follow-up information were obtained. The SEER registry provides detailed information regarding the pathological stage of LN involvement (pN), the number of positive LN and the number of LN examined during surgery. The NR was divided into low and high group, and all patients were further divided into 4 categories with different combinations of pN and NR.
The primary outcome of interest was overall survival (OS), which was calculated using the interval between surgery and death of all causes or the end of this study; secondary outcome was cause-specific survival (CSS), which was defined as time to death from lung cancer, with patients censored at the end of this study.
Statistical analysis
All categorical variables were presented as a percentage. Continuous variables were reported as either mean and standard deviation (SD) or median and interquartile range (IQR) depending on the distribution of data. The maximally selected log-rank statistics was used for the analysis of NR to find the optimal cutoff with best discriminative ability of survival. This analysis was performed with every 0.05 increment of NR. The pN-NR was investigated as the predictor of OS and CSS using Cox proportional hazards regression models (univariable and multivariable). Covariates included the following characteristics: age, race, gender, laterality, type of surgery, histological subtype, pTNM stage, pT stage, pN stage, NR and pN-NR. A multivariable model was developed using stepwise regression (forwards selection) by selecting significant variables upon univariable analysis. Enter limit and remove limit were P=0.10 and P=0.15, respectively.
Survival curves were plotted using the Kaplan-Meier method and the log-rank test was used to assess differences between pN-NR groups. Survival was also estimated after stratified by age, pT stage, type of surgery, and histological type to assess if prognostic differences across the 4 pN-NR groups remained significant after controlling for these confounders. The results of all survival models were presented as adjusted hazard ratios (HR) with 95% confidence interval (95% CI). Significance was defined by two-tailed P value<0.05. The SPSS software (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.) was used for all analyses. This study is based on the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline (12). The statistical analysis is based on the European Journal of Cardio-Thoracic Surgery and the Interactive Cardio Vascular and Thoracic Surgery guideline (13).
Results
From 2004 to 2012, 69,727 patients undergoing surgical resection for lung cancer with positive LN were identified from the SEER database. Among them 12,170 met the study criteria. Patient records including demographic, surgical and pathological information was presented in Table 1. The mean age of diagnosis was 64.8±9.2 years old. Histological analysis showed a predominance of adenocarcinoma (59.0%). The median numbers of resected LN and metastatic LN were 12 (IQR: 9–18) and 2 (IQR: 1–4), respectively. N1 and N2 LN metastasis were identified in 57.9% and 42.1% cases respectively.
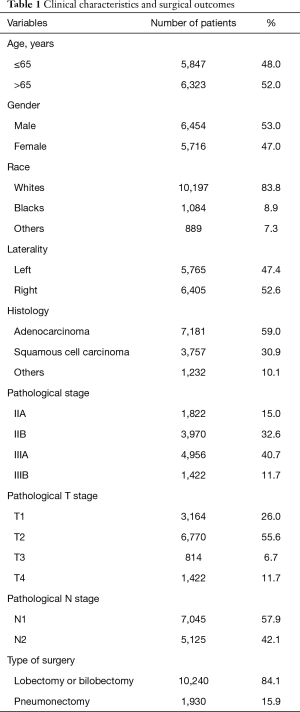
Full table
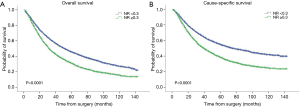
A total of 6,621 (54.4%) deaths were recorded by the time of this study and 5,039 (41.4%) deaths were caused by lung cancer. The 5-year OS and CSS were 41.7% and 50.7%. As for the relationship between NR and OS or CSS, the maximal χ2 value was reached when the NR setting was 0.3. So the NR was divided into low and high groups with this cut point, and all patients were further divided into 4 groups: pN1-NR <0.3, pN1-NR ≥0.3, pN2-NR <0.3 and pN2-NR ≥0.3.
In univariable analysis, age, gender, race, surgery type, histological type, pTNM stage, pT stage, pN stage, NR and pN-NR were predictive factors for both OS and CSS. In multivariable analysis, age, gender, race, surgery type, histological type, pT stage and pN-NR were independent prognostic factors for OS, and age, gender, surgery type, histological type, pT stage and pN-NR were independent prognostic factors for CSS (Table 2). Compared with those for the patients in the pN1-NR <0.3 group, the hazard ratio of OS was 1.405 (95% CI: 1.295–1.524), 1.183 (95% CI: 1.113–1.257) and 1.717 (95% CI: 1.607–1.835) times higher for patients in the pN1-NR ≥0.3, pN2-NR <0.3 and pN2-NR ≥0.3 groups, respectively, and 1.518 (95% CI: 1.385–1.665), 1.221 (95% CI: 1.138–1.310), 1.849 (95% CI: 1.715–1.993) times higher for CSS (Table 2).
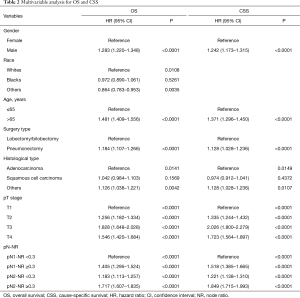
Full table
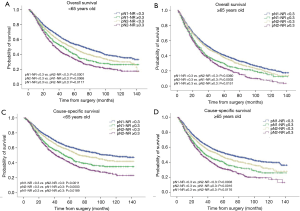
The survival curves showed that NR <0.3 was associated with better survival in the entire population, with 5-year OS 45.6% vs. 31.0% (P<0.0001) and 5-year CSS 55.1% vs. 38.1% (P<0.0001) (Figure 1). When stratified by the pN-NR, the survival curves separated well between the 4 groups, with 5-year OS 47.1% for pN1-NR <0.3, 43.0% for pN2-NR <0.3, 35.0% for pN1-NR ≥0.3 and 28.5% for pN2-NR ≥0.3. The differences in survival between neighboring pN-NR groups were statistically significant. The same trend went for CSS, with 5-year survival rate 56.8% for pN1-NR <0.3, 51.7% for pN2-NR <0.3, 43.3% for pN1-NR ≥0.3 and 35.6% for pN2-NR ≥0.3 (Figure 2).
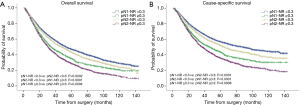
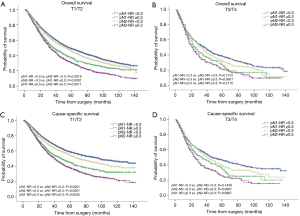
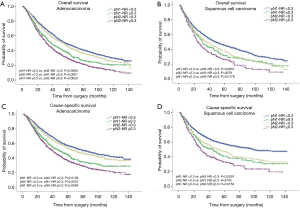
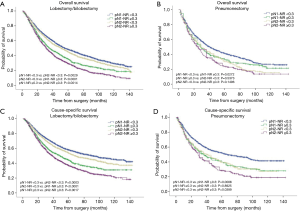
The prognostic role of pN-NR was further analyzed within subgroups. For patients with T1/T2 disease, adenocarcinoma, age <65 or ≥65, and lobectomy/bilobectomy, the best OS and CSS were reached in the pN1-NR <0.3 group, followed by the pN2-NR <0.3 group, the pN1-NR ≥0.3 group, and the pN2-NR ≥0.3 group in sequence, and the differences between neighboring pN-NR groups were statistically significant. For patients with T3/T4 disease, squamous cell carcinoma and pneumonectomy, the survival of the pN2-NR <0.3 group and the pN1-NR ≥0.3 group wasn’t statistically significant, though the pN2-NR <0.3 group was better except for pneumonectomy (Figures 3,4,5,6, Table 3).
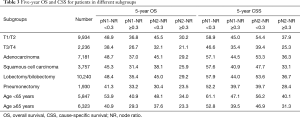
Full table
Discussion
The pathological status of LN is one of the most important prognostic factors for NSCLC after surgery. In the current TNM system, the assessment of nodal status has always only focused on the anatomic location of metastatic LN (3). However, some studies have shown that this nodal staging method is unsatisfactory in distinguishing the heterogeneous pN1 and pN2 NSCLC (2,14). As a result, some authors suggested that NR, which takes into account the number of resected LN and positive LN, could be a useful predictive factor for survival (7,15,16). In this study we used the population-based SEER database to assess the combined efficiency of node location and node ratio (pN-NR) for the prognosis of NSCLC after surgery.
In this study, patients were excluded whose number of resected LN was less than 6 to ensure relatively standard lymphadenectomy. This limit is recommended by the European Society of Thoracic Surgeons (17), and some other studies also used this limit (9,10). The value of NR could be significantly affected by extremums if only few or no LN was harvested. Another consideration is that there is evidence showing that inadequate LN resection is an independent factor for poorer survival, and we tried to eliminate this effect (6,18). Some studies also suggested 10 LN, even 16 LN for accurate assessment of nodal status (6,14). To keep a balance between the quality of surgery and study sample size, we set resection of 6 LN as the threshold for inclusion. The median number of resected LN in our study was 12. Actually, 45,047 cases were excluded for this single criterion in our study. So, we believe this criterion is crucial to ensure the quality of this study. Patients with pN0 were also excluded because it’s impossible to calculate the NR in these patients, and it is widely accepted that these patients have better survival than node positive patients (3). In this study we are unable to distinguish systemic LN dissection and LN sampling. However, since all the patients in this study were node positive and had at least 6 LN harvested, we believe that they received relatively sufficient LN dissection.
Some authors have investigated the effect of NR on survival for lung cancer, and the cut point of NR they determined ranged between 15–50% (15,19-21). There are two studies based on the SEER database and both have drawn the conclusion that a lower NR is associated with better survival (20,22). But these two studies didn’t restrict the minimum number of LN resected, and both collected data from a long-time span (1988 to 2007), which might result in significant patient selection bias. The management of lung cancer, including the preoperative staging method, neoadjuvant therapy and the extent of mediastinal lymphadenectomy could be different in the 1980s and 1990s. So, their study population was highly heterogeneous, and included a number of cases without adequate LN assessment. What’s more, the cut point of NR they chose was simply determined by the mathematical distribution of NR, and thus could result in significant bias. In our study, the cases selected were between 2004 to 2012 and with at least 6 LN resected. What’s more, we used the maximally selected log-rank statistics to find out the optimal cut point of NR, and thus we believe our result is more convincing.
The pN-NR evaluated in this study takes into consideration two important aspects of the status of metastasis LN, which are the anatomic location and the positive LN ratio. We found out that the survival curves of the different pN-NR groups separated well with each other for both OS and CSS, and this result was consistent in most subgroups. This means that the pN-NR has a powerful discriminative ability concerning the prognosis of NSCLC. So, we believe it could be an eligible index for the description of nodal status for NSCLC after surgery. What’s more, the pN-NR is easy to calculate and obtain.
The survival curves of the different pN-NR groups were not statistically significant in the T3/T4 subgroup, though a tendency towards the deterioration of OS and CSS was similar to that of the entire population. The reason may be that the prognosis of the higher pT category is already poor, regardless of the status of metastatic LNs. What’s more, the pT staging of the 6th edition used in this study is quite different from that of the current 8th edition, so the effectiveness of pN-NR for higher pT stage should be explored with the latest system (23). In patients undergoing pneumonectomy, the survival curves overlapped. This may be related to the surgery itself. Pneumonectomy is a major surgery, and the survival benefit for tumor resection could be significantly affected by the comorbidity of patients, while the node status is less important (24). But we are unable to obtain this information from the SEER database.
In this study, the patients in the pN2-NR <0.3 group had better OS and CSS than the patients in the pN1-NR ≥0.3 group, and subgroup analysis supported this finding in most occasions though in some cases the difference wasn’t statistically significant. It’s widely accepted that the pN2 patient group has worse prognosis than the pN1 patient group when other conditions are comparable. A Japanese investigation showed that the 5-year OS for pN1 and pN2 were 65.9% and 35.4%, respectively; the 5-year DFS for pN1 and pN2 were 75.3% and 31.1%, respectively (14). But our result indicated that the prognosis of pN1 and pN2 could be reversed when stratified by the pN-NR. This reflects a possible limitation of the present pN classification system for nodal status. There are several possible underlying reasons for this phenomenon. First, the NR seems to be a more powerful factor for survival. A higher NR could indicate a more aggressive malignant behavior, or higher LN tumor burden, which can exceed the effect of metastatic LN station. Several studies have a similar finding. In the study by Ding et al. involving 700 node positive NSCLC patients, the NR was a superior prognostic factor (8). A Japanese research involving 437 pN0, 113 pN1 and 101 pN2 patients reported that LNR followed by nN may be a more effective prognostic indicator than pN (25). Second, the patients in the pN1-NR ≥0.3 group may be understaged to pN1 due to insufficient lymphadenectomy. The correlation between the number of examined LN and stage migration has been reported (6). Since all patients in this study were node positive, it’s possible that a relatively high NR in the pN1-NR ≥0.3 group could result from a relatively small number of resected LN, thus potential positive N2 could be missed out. The undiscovered N2 disease may discourage patients from receiving proper adjuvant therapy and active follow-up, and thus result in compromised OS and CSS. Another study also proved that a small number of resected LN is directly associated with a worse survival outcome in NSCLC patients (26). Finally, the patients in the pN2-NR <0.3 group are more likely to have lobe-specific metastatic or skip-N2 disease, or single-station N2 disease, which was reported to have better survival than pN2 disease in general (27-29). This also reflects that N2 is a highly heterogeneous disease group which needs to be further stratified under the current TNM system, and the pN-NR could be a promising index.
The NR has been used to stratify patients with different recurrent risks and guide treatment (30). We believe that the pN-NR could also be used to guide treatment. A study in 2016 using the National Cancer Database found out that only 53% of the pN1 patients received peri-operative chemotherapy though it is recommended by established guidelines (31). This may be due to the conception that pN1 patients have better survival than pN2 patients, and the concerns about the severe adverse effects of chemotherapy. But according to our result, for the pN1 patients with NR ≥0.3, the OS and CSS were worse than the pN2 patients with NR <0.3. According to this result, maybe aggressive adjuvant therapy should be considered for pN1-NR ≥0.3 patients, and this needs to be further explored.
The limitations of this study are attributable to the retrospective nature and the inherent limitations from using the SEER database. The SEER database lacks granular details such as smoking history, comorbidity, clinical staging, peri-operative therapy, LN station dissected and recurrence patterns (32). So, the results of this study should not be over-interpreted given the inherent weaknesses in SEER analyses in general. Neoadjuvant chemotherapy could lead to LN down-stage. However, according to the EMERGING-CTONG 1103 trial, the LN down-stage occurred in only 2.9% patients in the neoadjuvant chemotherapy group (33). So, we believe that the pre-operative chemotherapy could have very limited effect on our result. Another relevant point is that we collected cases over a long time period, and the surgical techniques regarding LN dissection could be different in recent years compared with those of earlier times. In addition, two possible biases could lead to a miscount of the LN number: underestimation as a result of the difficulty in separating each LN in the dissected tissues and overestimation because of fragmentation of nodal tissues during the removal of LNs. All these factors could complicate the interpretation of results. What’s more, right now it is impossible to accurately estimate the number of metastatic LN for both pre-operative and inoperable NSCLC through any diagnostic methods, so the pN-NR could only be applied for the p stage.
Conclusions
This study reveals that the pN-NR could be a good predictor for the prognosis of NSCLC after curative resection and 0.3 is the optimal cut point of NR. The finding has the potential to act as a useful supplement to the current TNM staging system and guide adjuvant therapy. Prospective studies are needed to validate the effectiveness of pN-NR classification for NSCLC in the future.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-758
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-758
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-758). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval for this study and informed consent were waived from the ethics committee in the China-Japan Friendship Hospital due to the retrospective and public database nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Wei S, Asamura H, Kawachi R, et al. Which is the better prognostic factor for resected non-small cell lung cancer: the number of metastatic lymph nodes or the currently used nodal stage classification? J Thorac Oncol 2011;6:310-8. [Crossref] [PubMed]
- Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84.
- Donohoe CL, Phillips AW. Cancer of the esophagus and esophagogastric junction: an 8(th) edition staging primer. J Thorac Dis 2017;9:E282-4. [Crossref] [PubMed]
- Giuliano AE, Connolly JL, Edge SB, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:290-303.
- Liang W, He J, Shen Y, et al. Impact of Examined Lymph Node Count on Precise Staging and Long-Term Survival of Resected Non-Small-Cell Lung Cancer: A Population Study of the US SEER Database and a Chinese Multi-Institutional Registry. J Clin Oncol 2017;35:1162-70. [Crossref] [PubMed]
- Taylor MD, LaPar DJ, Thomas CJ, et al. Lymph node ratio predicts recurrence and survival after R0 resection for non-small cell lung cancer. Ann Thorac Surg 2013;96:1163-70. [Crossref] [PubMed]
- Ding X, Hui Z, Dai H, et al. A Proposal for Combination of Lymph Node Ratio and Anatomic Location of Involved Lymph Nodes for Nodal Classification in Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:1565-73. [Crossref] [PubMed]
- Pawełczyk K, Marciniak M, Blasiak P. Evaluation of new classifications of N descriptor in non-small cell lung cancer (NSCLC) based on the number and the ratio of metastatic lymph nodes. J Cardiothorac Surg 2016;11:68. [Crossref] [PubMed]
- Chiappetta M, Leuzzi G, Sperduti I, et al. Lymph-node ratio predicts survival among the different stages of non-small-cell lung cancer: a multicentre analysis. Eur J Cardiothorac Surg 2019;55:405-12. [Crossref] [PubMed]
- Cronin KA, Ries LA, Edwards BK. The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. Cancer 2014;120 Suppl 23:3755-7. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495-9. [Crossref] [PubMed]
- Hickey GL, Dunning J, Seifert B, et al. Statistical and data reporting guidelines for the European Journal of Cardio-Thoracic Surgery and the Interactive CardioVascular and Thoracic Surgery. Eur J Cardiothorac Surg 2015;48:180-93. [Crossref] [PubMed]
- Saji H, Tsuboi M, Shimada Y, et al. A proposal for combination of total number and anatomical location of involved lymph nodes for nodal classification in non-small cell lung cancer. Chest 2013;143:1618-25. [Crossref] [PubMed]
- Qiu C, Dong W, Su B, et al. The prognostic value of ratio-based lymph node staging in resected non-small-cell lung cancer. J Thorac Oncol 2013;8:429-35. [Crossref] [PubMed]
- Kang CH, Ra YJ, Kim YT, et al. The impact of multiple metastatic nodal stations on survival in patients with resectable N1 and N2 nonsmall-cell lung cancer. Ann Thorac Surg 2008;86:1092-7. [Crossref] [PubMed]
- De Leyn P, Lardinois D, Van Schil P, et al. European trends in preoperative and intraoperative nodal staging: ESTS guidelines. J Thorac Oncol 2007;2:357-61. [Crossref] [PubMed]
- Fukui T, Mori S, Yokoi K, et al. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol 2006;1:120-5. [Crossref] [PubMed]
- Wang CL, Li Y, Yue DS, et al. Value of the metastatic lymph node ratio for predicting the prognosis of non-small-cell lung cancer patients. World J Surg 2012;36:455-62. [Crossref] [PubMed]
- Nwogu CE, Groman A, Fahey D, et al. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann Thorac Surg 2012;93:1614-9; discussion 1619-20. [Crossref] [PubMed]
- Wisnivesky JP, Arciniega J, Mhango G, et al. Lymph node ratio as a prognostic factor in elderly patients with pathological N1 non-small cell lung cancer. Thorax 2011;66:287-93. [Crossref] [PubMed]
- Jonnalagadda S, Arcinega J, Smith C, et al. Validation of the lymph node ratio as a prognostic factor in patients with N1 nonsmall cell lung cancer. Cancer 2011;117:4724-31. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Doddoli C, Barlesi F, Trousse D, et al. One hundred consecutive pneumonectomies after induction therapy for non-small cell lung cancer: an uncertain balance between risks and benefits. J Thorac Cardiovasc Surg 2005;130:416-25. [Crossref] [PubMed]
- Matsuguma H, Oki I, Nakahara R, et al. Proposal of new nodal classifications for non-small-cell lung cancer based on the number and ratio of metastatic lymph nodes. Eur J Cardiothorac Surg 2012;41:19-24. [PubMed]
- Wen YS, Xi KX, Xi KX, et al. The number of resected lymph nodes is associated with the long-term survival outcome in patients with T2 N0 non-small cell lung cancer. Cancer Manag Res 2018;10:6869-77. [Crossref] [PubMed]
- Li H, Hu H, Wang R, et al. Lung adenocarcinoma: Are skip N2 metastases different from non-skip? J Thorac Cardiovasc Surg 2015;150:790-5. [Crossref] [PubMed]
- Sun Y, Gao W, Zheng H, et al. Mediastinal lymph-nodes metastasis beyond the lobe-specific: an independent risk factor toward worse prognoses. Ann Thorac Cardiovasc Surg 2014;20:284-91. [Crossref] [PubMed]
- Wang L, Zhan C, Gu J, et al. Role of Skip Mediastinal Lymph Node Metastasis for Patients With Resectable Non-small-cell Lung Cancer: A Propensity Score Matching Analysis. Clin Lung Cancer 2019;20:e346-55. [Crossref] [PubMed]
- Shang X, Li Z, Lin J, et al. PLNR ≤20% may be a benefit from PORT for patients with IIIA-N2 NSCLC: a large population-based study. Cancer Manag Res 2018;10:3561-7. [Crossref] [PubMed]
- Bott MJ, Patel AP, Verma V, et al. Patterns of care in hilar node-positive (N1) non-small cell lung cancer: A missed treatment opportunity? J Thorac Cardiovasc Surg 2016;151:1549-58.e2. [Crossref] [PubMed]
- Varlotto JM, Recht A, Nikolov M, et al. Extent of lymphadenectomy and outcome for patients with stage I nonsmall cell lung cancer. Cancer 2009;115:851-8. [Crossref] [PubMed]
- Zhong WZ, Chen KN, Chen C, et al. Erlotinib Versus Gemcitabine Plus Cisplatin as Neoadjuvant Treatment of Stage IIIA-N2 EGFR-Mutant Non-Small-Cell Lung Cancer (EMERGING-CTONG 1103): A Randomized Phase II Study. J Clin Oncol 2019;37:2235-45. [Crossref] [PubMed]


