Endobronchial ultrasound with a guide sheath for small malignant pulmonary nodules: a retrospective comparison between central and peripheral locations
Introduction
Current guidelines in the management of a peripheral pulmonary nodule (PPN) recommend bronchoscopy as one of the non-surgical diagnostic procedures with a more favourable safety profile (1). The addition of various image-guided modalities, one of which is radial endobronchial ultrasound with a guide sheath (EBUS-GS), has improved the diagnostic yield of transbronchial biopsy (TBB) (2-4). Quite a number of researches have assessed the utility of EBUS-GS during TBB and by far, an EBUS probe location that is within the lesion has been the most consistent factor associated with a high accuracy (5-8).
Some issues that can help maximize the benefits of TBB with EBUS-GS have not been unanimously settled yet (9). The effect of lesion size, location, or size of sampling device on diagnostic performance varies among studies (5-8,10-12). Seemingly, a better diagnostic yield would be expected for larger nodules that are closer to the central airways and if biopsy samples are larger, but this hypothesis should be supported by objective data.
Essentially, refinements are needed to help clinicians choose an approach that is best suited for a particular patient. In this study, we aimed to investigate the factors affecting diagnostic yield of TBB with EBUS-GS for small malignant PPNs and to know what group of patients can benefit most.
Materials and methods
Study design and population
A retrospective review of electronic medical records at the National Cancer Center, Tokyo was carried out on consecutive cases of PPNs that underwent TBB with EBUS-GS at the Respiratory Endoscopy division of the hospital, from April 2012 to March 2013.
Cases that had a final diagnosis of malignancy were included in the study population. Those that were benign or had uncertain diagnosis after one year of follow-up and cases that necessitated removal of the GS during sampling were excluded.
Informed consent was sought from every patient and this study was approved by the Institutional Review Board of the hospital.
Study variables and statistical analysis
Data on demographics, radiologic characteristics, procedural findings, and final diagnoses were collected. PPN was defined as an abnormal lung parenchymal lesion measuring ≤30 mm in largest diameter on axial plane CT scan and that was not visible endoscopically. CT scan characteristics were classified as solid or ground glass opacity (GGO). Location in the pulmonary parenchyma was decided based on a previous study and was designated as “central parenchymal” if the nodule was not adjacent to the costal visceral pleura; or “peripheral parenchymal” if the nodule was adjacent to, or within 10 mm from the costal visceral pleura (13). Lobe was recorded as upper, middle/lingula, and lower.
Data gathered from the bronchoscopy procedure were GS size, EBUS probe location, number of tissue samples, and procedure time (from vocal cord insertion to removal of the GS). Primary endpoint was diagnostic accuracy. The study population was divided according to location and examined separately for factors affecting diagnostic yield.
Data was analysed using IBM SPSS Statistics Software Version 21. Frequencies were presented as mean with standard deviation (SD) and percentages; continuous variables were categorized. Univariate analysis was by Fisher’s exact test and Pearson chi-square. Multivariate analysis was by logistic regression. A P value of ≤0.05 was considered significant. Sub-group analysis was performed by crosstabs.
EBUS-GS transbronchial sampling procedure
Procedures were performed at the Respiratory Endoscopy Unit of the hospital; most of the time, the operator was a resident/fellow trainee, under the direct supervision of experienced staff members. Bronchoscopy was performed through the oral route under local anesthesia with conscious sedation. For all cases, the bronchial path to the target lesion was planned using 1-5 mm sequential axial CT scan slices. The choice of bronchoscope and devices (all by Olympus, Tokyo, Japan) depended on each case and availability of equipment. The BF 1T260 (5.9 mm outer diameter, 2.8 mm working channel diameter) was used with a UM-S20-20R radial EBUS probe and a large GS Kit (K-203). The BF-Type260 (5.0 mm outer diameter, 2.0 mm working channel diameter) or P260F (4.0 mm outer diameter, 2.0 mm working channel diameter) was used with a UM-S20-17S radial EBUS probe and a small GS Kit (K-201). Fluoroscopy (VersiFlex VISTA, Hitachi, Japan) was used intermittently during each procedure; specifically during tumor localization by EBUS-GS, during sampling, and during removal of the GS after sampling.
The devices were prepared and the sampling site was searched as usual (2,6). To locate the target site, we used the typical radial EBUS images that have been previously described for solid nodules (6) and the Blizzard sign for GGO nodules (14). When the EBUS probe was found to be within the lesion, it was removed from the GS and TBB was started. When the EBUS probe was adjacent to the lesion or invisible, it was adjusted until the closest possible area to the target site was reached.
Specimens for pathology examination were obtained by alternately inserting biopsy forceps and cytology brush through the GS. Rapid on-site evaluation (ROSE) was performed by an experienced cytopathologist. For PPNs that did not have a “within” location of EBUS probe or when ROSE showed inadequate specimen, transbronchial needle aspiration (TBNA) was done using a 21-G aspiration needle (NA-1C-1) through a large GS (15). In cases wherein the initial GS size used was small, the GS was removed prior to TBNA.
The final diagnoses were established by pathologic evidence from bronchoscopic or surgical biopsy, microbiological analysis, or clinical follow-up.
Results
The study population consisted of 212 patients with a mean age of 67.9 (SD 10.47) years and with PPNs measuring 20.45 (SD 5.45 mm) (Table 1). There were 91 nodules that were “peripheral parenchymal”, while 121 nodules were “central parenchymal” in location. Overall diagnostic yield of EBUS-GS was 67.5% and majority were adenocarcinoma. There were no major post-procedural complications.
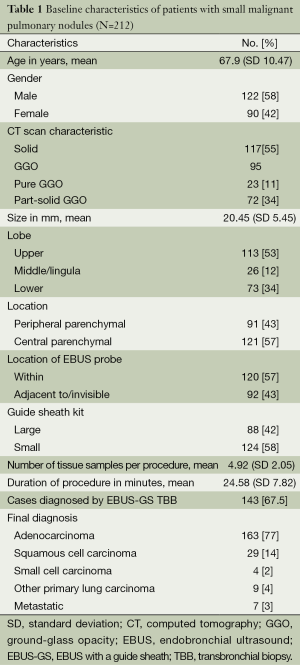
Full table
Table 2 shows the factors affecting diagnostic yield of EBUS-GS TBB for small malignant parenchymal nodules. The diagnostic yield for central parenchymal lesions was significantly higher than that for peripheral parenchymal lesions (77% vs. 55%, P=0.001). In the univariate analysis, lesions wherein the EBUS probe could be placed within had a significantly higher diagnostic yield compared to when the EBUS probe was adjacent or invisible (68% vs. 54%, P=0.001). In the multivariate analysis, central parenchymal location and EBUS probe within were the predictors of a successful TBB. Diagnostic yield was the same regardless of demographics, nodule size, CT scan characteristic, lobar location, or GS kit size used.
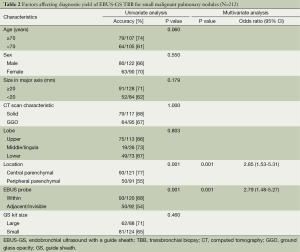
Full table
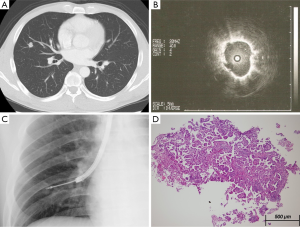
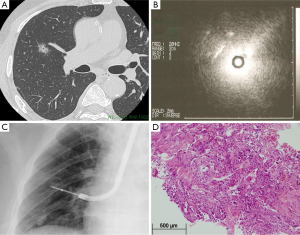
A sub-group analysis that compares the diagnostic accuracy between peripheral parenchymal and central parenchymal locations based on nodule and procedural characteristics is shown in Table 3. The diagnostic accuracy of EBUS-GS TBB was highest (87%) for central parenchymal lesions that had an EBUS probe within. Representative cases are presented in Figures 1 and 2. In addition, the diagnostic yield for central parenchymal was at least 75% and significantly higher than that of peripheral parenchymal when the lesion was <20 mm in size, solid on CT scan, located in the upper and lower lobes, and when a small size GS kit was used.
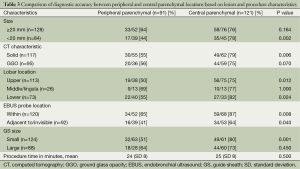
Full table
Discussion
Endobronchial ultrasound has undeniably contributed a great deal to the recent advancement of diagnostic bronchoscopy for PPNs. Systematic reviews on radial EBUS for PPNs have reported an overall diagnostic accuracy of 71%, though with significant inter-study heterogeneity and yields varying widely from 46-86 percent (4,5). With additional use of a GS, localization of the lesion has consistently translated to better diagnostic yields ranging from 73 to 92 percent (5-8).
This one-year study on 212 small malignant PPNs demonstrated that TBB with EBUS-GS has an overall diagnostic performance of 67.5 percent. Our data is consistent with previously published reports (5-7,9,16) that an EBUS probe within was a significant predictor of procedural success. Proximity to the hilum is an important feature mentioned in literature; accuracy of the procedure for lesions that were touching the visceral pleura was only 35-50 percent (5,7,11). From our analysis, an easily accessible lesion (central parenchymal) was more likely to be diagnosed successfully than a more distally located lesion. For central parenchymal nodules, diagnostic accuracy increased significantly to 77 percent. This difference in diagnostic yield between peripheral and central parenchymal locations was significant especially for lesions <20 mm in size, solid in character, located in the upper and lower lobes, and when the GS kit used was small. When combined with an EBUS probe that could be precisely localized within the lesion, TBB with EBUS-GS for central parenchymal lesions had a remarkably higher yield of 87 percent. The average number of TBB samples was five per procedure. There were no major complications.
The findings of this study could be helpful when choosing a diagnostic modality for clinically suspected malignant PPNs that are away from the pleura. Transthoracic needle aspiration (TTNA) has a similar diagnostic yield for PPNs but with higher accompanying risks (1,17,18). Our results could also be important for beginner physicians. At the start of the learning curve, it might be prudent to perform TBB with EBUS-GS for patients who are more likely to be diagnosed accurately. For lesions that are adjacent to the pleura, use of virtual bronchoscopic navigation (VBN) may be useful to increase the yield (19).
Nodule size has been cited by some studies (1,4,5,18) to significantly influence diagnostic yield but other researches present opposing results (6,12,20). Also, we hypothesized that using a large GS and its corresponding sampling devices is preferred, especially for GGOs. This study demonstrated that TBB with EBUS-GS is an acceptable diagnostic modality for malignant PPNs regardless of size, CT scan characteristic (solid or GGO), lobar location, or GS kit size.
Our study has some limitations. First, although TBNA was used in some cases in this study population, we did not have sufficient reliable data to include this variable in the analysis. Second, the unequal distribution of patients in each of the subgroups (Table 3) should be taken into consideration when analysing these results. Last, it may be noteworthy that our follow-up period was less than the recommended time frame to establish stability of both solid and GGO nodules; thus, false-negative results are possible. Since this was a retrospective, single-center research, we suggest prospective randomized controlled studies in the future.
Nevertheless, this research highlights that precise search of a biopsy site using EBUS is essential when performing TBB for small peripheral lung cancer. Patients with lesions that are not adjacent to the costal visceral pleura may potentially benefit more from the procedure.
Conclusions
EBUS-GS as an aide during TBB has an acceptable diagnostic yield for small malignant PNs. The value of the procedure can be maximized for patients who have parenchymal lesions that are not adjacent to the pleura and can be precisely localized by the radial EBUS probe.
Acknowledgements
This work was supported by the National Cancer Center Research and Development Fund (25-A-12). The primary author also wishes to extend her gratitude to Takeda Science Foundation.
Authors’ contributions: C Chavez and S Sasada designed the overall study with significant contributions from T Izumo, T Tsuchida, C Chavez, J Watanabe, M Katsurada and Y Matsumoto all substantially contributed to the acquisition, interpretation, and consolidation of data. Statistical analysis was performed by C Chavez and T Izumo. The manuscript was written by C Chavez, was revised critically for important intellectual content by S Sasada, and reviewed by T Izumo, J Watanabe, M Katsurada, Y Matsumoto, and T Tsuchida. All authors approved of the final version of the manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J 2002;20:972-4. [PubMed]
- Ishida M, Suzuki M, Furumoto A, et al. Transbronchial biopsy using endobronchial ultrasonography with a guide sheath increased the diagnostic yield of peripheral pulmonary lesions. Intern Med 2012;51:455-60. [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [PubMed]
- Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011;37:902-10. [PubMed]
- Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959-65. [PubMed]
- Huang CT, Ho CC, Tsai YJ, et al. Factors influencing visibility and diagnostic yield of transbronchial biopsy using endobronchial ultrasound in peripheral pulmonary lesions. Respirology 2009;14:859-64. [PubMed]
- Tamiya M, Okamoto N, Sasada S, et al. Diagnostic yield of combined bronchoscopy and endobronchial ultrasonography, under LungPoint guidance for small peripheral pulmonary lesions. Respirology 2013;18:834-9. [PubMed]
- Akulian J. Bridging the gap between localization and diagnosis of peripheral pulmonary nodules. Respirology 2013;18:739-40. [PubMed]
- Tay JH, Irving L, Antippa P, et al. Radial probe endobronchial ultrasound: factors influencing visualization yield of peripheral pulmonary lesions. Respirology 2013;18:185-90. [PubMed]
- Fielding DI, Robinson PJ, Kurimoto N. Biopsy site selection for endobronchial ultrasound guide-sheath transbronchial biopsy of peripheral lung lesions. Intern Med J 2008;38:77-84. [PubMed]
- Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:36-41. [PubMed]
- Sasada S, Izumo T, Chavez C, et al. A new middle-range diameter bronchoscope with large channel for transbronchial sampling of peripheral pulmonary lesions. Jpn J Clin Oncol 2014;44:826-34. [PubMed]
- Sasada S, Izumo T, Chavez C, et al. Blizzard sign as a specific endobronchial ultrasound image for ground glass opacity: a case report. Respiratory Medicine Case Reports 2014;12:19-21.
- Takai M, Izumo T, Chavez C, et al. Transbronchial needle aspiration through a guide sheath with endobronchial ultrasonography (GS-TBNA) for peripheral pulmonary lesions. Ann Thorac Cardiovasc Surg 2014;20:19-25. [PubMed]
- Izumo T, Sasada S, Chavez C, et al. The diagnostic utility of endobronchial ultrasonography with a guide sheath and tomosynthesis images for ground glass opacity pulmonary lesions. J Thorac Dis 2013;5:745-50. [PubMed]
- Tomiyama N, Yasuhara Y, Nakajima Y, et al. CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol 2006;59:60-4. [PubMed]
- Fielding DI, Chia C, Nguyen P, et al. Prospective randomised trial of endobronchial ultrasound-guide sheath versus computed tomography-guided percutaneous core biopsies for peripheral lung lesions. Intern Med J 2012;42:894-900. [PubMed]
- Asano F, Shinagawa N, Ishida T, et al. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy. A randomized clinical trial. Am J Respir Crit Care Med 2013;188:327-33. [PubMed]
- Kikuchi E, Yamazaki K, Sukoh N, et al. Endobronchial ultrasonography with guide-sheath for peripheral pulmonary lesions. Eur Respir J 2004;24:533-7. [PubMed]


