Perioperative fluid balance and 30-day unplanned readmission after lung cancer surgery: a retrospective study
Introduction
Restrictive fluid administration protocol, which aims for “zero fluid balance (FB),” has been reported to reduce postoperative complications and shorten hospital stay (1,2). Particularly, fluid overload is a risk factor for acute lung injury after surgeries such as lung resection (3-5), and thus, restrictive fluid administration, which aims for avoiding positive FB and targeting zero FB, has been increasingly emphasized (6). However, Wu et al. recently reported that excessive fluid restriction during surgery, as well as fluid overload, is associated with elevated postoperative complications after minimally invasive lobectomy of the lung (7). This is an important issue in relation to the fact that excessive fluid restriction induces tissue hypoperfusion and reduces end-organ perfusion (8). In other words, either positive or negative FB may increase complications as well as lengthen hospital stay after thoracic surgery.
Hospital readmissions after hospitalization are known as an associated factor with higher resource utilization and worse patient outcomes (9). A recent report stated that annual cost related to unplanned readmission is $17.4 billion in the United States (10). Postoperative readmission accounts for a significant percentage of unplanned hospital readmission after discharge, and according to a recent report, 30-day unplanned readmission after surgery is about 5.7% in the United States (11). Although multiple factors are associated with 30-day unplanned readmission, a surgical complication has been reported as an important factor that potentially increases readmission (12-14). Thus, lowering surgical complications and 30-day unplanned readmission rate in the perioperative period is crucial and is a challenging issue to ensure the quality of care and cost reduction for surgical population. Because perioperative FB is reported to be associated with a higher postoperative complication rate after thoracic surgery (7), there might be an association between perioperative FB and 30-day unplanned readmission rate after thoracic surgery. However, adequate information is unavailable to validate this association.
Therefore, this study aims to investigate whether FB status during and up to 24 hours after lung resection surgery is associated with 30-day unplanned readmission rate. We hypothesized that both perioperative positive and negative FB status would be associated with an increase of 30-day unplanned readmission rate. This retrospective observational study was performed in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1474).
Methods
Ethical statement and study design
This study also conformed to the provisions of the Declaration of Helsinki (as revised in 2013). This retrospective observational study was approved by the institutional review board (IRB) of the Seoul National University Bundang Hospital (SNUBH) (IRB approval number: B-1903/528-101; approval date: February 25, 2019). The requirement to obtain informed consent was exempted in consideration of the retrospective design, where medical records are reviewed after completion of care.
Study population
We reviewed the medical records of patients aged 19 years or older who underwent curative resection after being diagnosed with primary non-small cell lung cancer (NSCLC) at the SNUBH between January 2005 and February 2018. The exclusion criteria were as follows: (I) patients with repeat lung surgery due to recurrent NSCLC were excluded, because we focused on primary lung cancer and not on recurrent lung cancer; (II) patients who underwent re-surgery within 24 hours after lung cancer surgery were excluded, because we focused on perioperative FB status during and up to 24 hours after lung cancer surgery; (III) patients who died during hospitalization after surgery were excluded considering the study design to determine the association between perioperative FB for lung cancer surgery and 30-day unplanned readmission; and (IV) patients with incomplete or missing data were also excluded from the analysis.
Cumulative FB during and up to 24 hours after surgery (independent variable)
FB was calculated during and up to 24 hours after surgery, because the length of hospital stay of patients with relatively simple lung surgery such as wedge resection was relatively short (2–3 days), and it was used in the RELIEF trial to define fluid management strategy (15). We used the equation reported in previous studies to compute cumulative FB (%) (16,17):
Cumulative Fluid Balance (%) = (Cumulative Fluid Input – Output) in liters × 100/Hospital Admission Weight (kg)
For example, 5% positive in a patient whose body weight was 50 kg means 2,500 mL positive FB. All types of intravenous and enteral fluids used for maintenance and resuscitation were included in the input fluid: colloids, crystalloids, blood products, drug infusions, and enteral and parenteral nutrition. The amount of drugs that were infused during and up to 24 hours after lung cancer surgery was also included in the calculation of perioperative FB. During surgery, remifentanil was usually infused by continuous infusion using a syringe pump, while rocuronium or cisatracurium was infused by bolus injection. All types of body fluids were included in the output fluid: estimated blood loss (mL); output from drains; rectal, orogastric, and nasogastric output; and urine. According to the standard in previous studies (18), patients were divided into the positive (>5%), normal (0–5%), and negative (<0%) groups on the basis of the cumulative FB computed as above.
Perioperative fluid management for lung cancer surgery
In SNUBH, fluid management during surgery was performed by anesthesiologists during the study period, and a balanced crystalloid was used as the main fluid according to our protocol. However, hydroxyethyl starch was sometimes used for fluid resuscitation according to the discretion of anesthesiologists in situations such as acute hypovolemic shock during surgery. After surgery, fluid management was performed by the thoracic surgeons. Although there was no specific protocol or guidelines for fluid management in the intraoperative and postoperative period, most anesthesiologists and thoracic surgeons in our hospital made efforts to achieve zero FB during the perioperative period for patients who underwent lung cancer surgery in order to avoid excessive fluid overload during the study period.
Thirty-day unplanned readmission (dependent variable)
All cases of readmission within 30 days of discharge after lung cancer surgery were defined as total 30-day readmission. From them, cases excluding routine evaluation according to the protocol of the surgical department and elective readmission for chemotherapy and radiotherapy were defined as 30-day unplanned readmission.
Measurements (potential covariates)
The following data were extracted as covariates for this study: physical characteristics [age, body mass index (kg·m−2), sex], calculated distance from home to hospital based on ZIP code (km), socioeconomic status-related information [insurance type (National health insurance program/medical aid beneficiary program), marital status (never married/married or living together/divorced or separated/widowed), occupation (office worker/licensed job/house work/self-employed/student, military, laborer, or unemployed), final educational attainment (lower than high school/more than or equal to high school, lower than college/more than or equal to college)], preoperative comorbidities [American Society of Anesthesiologists’ physical status, hypertension, diabetes mellitus, coronary artery disease (from stable angina to myocardial infarction), cerebrovascular disease, chronic obstructive pulmonary disease, tuberculosis, asthma, chronic kidney disease, anemia, heart failure, and dyslipidemia], operative data [video-assisted thoracoscopic surgery, surgery time, intraoperative remifentanil and rocuronium dosage, epidural analgesia, propofol-based total intravenous anesthesia, hydroxyethyl starch use, estimated blood loss, transfusion of packed red blood cells, type of surgery (lobectomy, sleeve lobectomy/wedge resection, segmentectomy/bilobectomy, pneumonectomy)], and histology of NSCLC (squamous cell carcinoma/adenocarcinoma/large cell, sarcomatoid type, and mixed type). Patients in the medical aid beneficiary program are those who are classified to have low income, and most of their hospital charges are paid by the government. Furthermore, for the patients in the national health insurance program, approximately two-thirds of their hospital charges are covered by the government. We included the year of surgery as a covariate for three reasons: (I) surgical techniques and anesthetic management used in our institution have improved from 2005 to 2018; (II) a previous study in 2013 reported that hydroxyethyl starch use might be associated with a higher risk of acute kidney injury, and this could affect the daily practice in our institution (19); and (III) the disease severity of patients who underwent lung cancer surgery in our institution might vary according to the year of surgery.
Study endpoint
The primary endpoint of this study was 30-day unplanned readmission after lung cancer surgery.
Statistical analysis
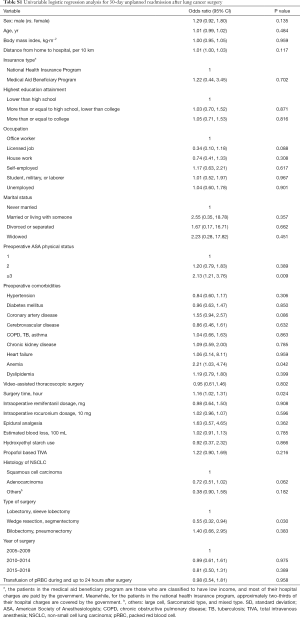
Baseline characteristics of the patients are presented as mean values with standard deviations (SDs) or as numbers with percentages. The simple relationship between the cumulative FB during and up to 24 hours after surgery was evaluated using restricted cubic splines. First, we performed a univariable logistic regression analysis to examine the individual associations between 30-day unplanned readmission and all covariates. Next, by using the criterion of P<0.2, we selected covariates from the univariable logistic regression analysis for the final multivariable logistic regression model. Considering the possibility of multicollinearity, continuous variables and categorical variables (positive, normal, and negative group) for cumulative FB were included in separate multivariable models. The goodness of fit of each multivariate model was tested with the Hosmer-Lemeshow test. The results of the logistic regression model are presented as odds ratios (ORs) with 95% confidence intervals (CIs). All analyses were performed using R version 3.6.1 (R Foundation, Vienna, Austria), and P<0.05 was deemed statistically significant.
Results
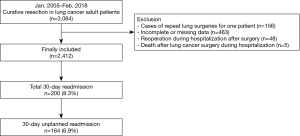
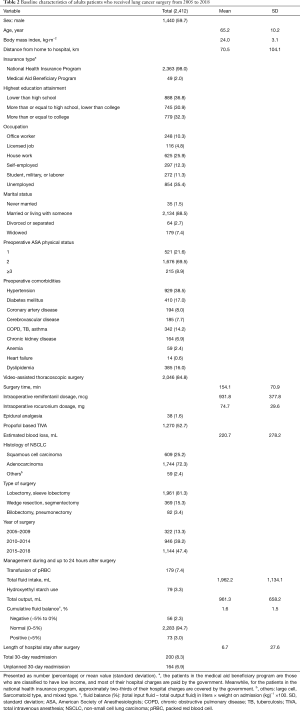
Between January 2005 and February 2018, 3,084 patients aged 19 years or older underwent curative resection for primary lung cancer. From them, 156 patients who underwent repeat lung surgeries due to recurrence, 463 patients with incomplete or missing data, 48 patients who underwent reoperation during hospital stay after initial surgery, and 5 patients who died during hospital stay after surgery were excluded, resulting in a total of 2,412 patients in the final analysis. From these patients, 200 had total 30-day readmission after discharge (8.3%, 200/2,412), 164 of whom had unplanned readmission (6.9%, 164/2,412) (Figure 1). The chief complaints of patients who had 30-day unplanned readmission are presented in Table 1, and the most common reason for unplanned readmission was pulmonologic symptoms (58.5%, 96/164). The baseline characteristics of all patients included in the study are shown in Table 2. The mean (standard deviation) perioperative cumulative FB of patients who underwent lung cancer surgery was 1.6% (1.5%). A total of 2,283 (94.7%) patients had normal cumulative FB, 56 (2.3%) had negative FB, and 73 (3.0%) had positive FB.

Full table
Full table
Cumulative FB (%) and 30-day unplanned readmission
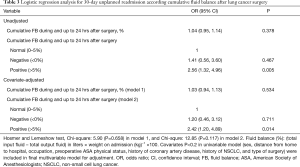
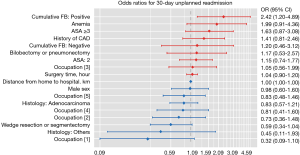
Figure S1 shows the log odds of 30-day unplanned readmission according to cumulative FB (%); as shown here, a linear relationship was observed between cumulative FB and 30-day readmission, and the log odds of 30-day unplanned readmission gradually increased according to cumulative FB. Table 3 shows the results of univariable and multivariable logistic regression analyses for 30-day unplanned readmission before and after adjusting for covariates with P<0.2 in Table S1. In the multivariable model, 1% increase of perioperative cumulative FB was not significantly associated with 30-day unplanned readmission (P=0.534; model 1). Compared to the normal FB group, the positive FB group had 2.42 times higher 30-day unplanned readmission (OR: 2.42, 95% CI: 1.20, 4.89; P=0.014; model 2), while the negative FB group did not significantly differ from the normal FB group (P=0.711). ORs with 95% CI of all individual variables in the final multivariable model (model 2) are presented as a forest plot in Figure 2, and with the exception of the positive FB group, all variables were not significantly associated with 30-day unplanned readmission (P>0.05).

Full table
Full table
Discussion
This retrospective cohort study found that positive FB (>5%) during and up to 24 hours after surgery was associated with higher 30-day unplanned readmission rates after lung cancer surgery. However, negative FB (<0%) was not significantly associated with 30-day unplanned readmission rates compared to normal FB. Our findings are meaningful because our study is the first to clarify the association between perioperative positive FB and the 30-day unplanned readmission rate after lung cancer surgery.
The present study showed that 1% increase of perioperative FB during and up to 24 hours after surgery was not associated with 30-day unplanned readmission rates after lung cancer surgery, while positive FB (>5%) during and up to 24 hours after surgery was associated with higher 30-day unplanned readmission rates after lung cancer surgery. These finding suggest that perioperative positive FB during and up to 24 hours after surgery needs to be evaluated with the criterion of >5% in future studies on unplanned readmission rate for lung cancer surgery.
Our results should be understood in light of the recent findings of Wu et al. (7). Wu et al. reported that both liberal fluid infusion rate and restrictive fluid infusion rate are associated with the increased incidence of postoperative pulmonary complications. One difference in our study is that we included open thoracotomy as well as minimally invasive surgeries and that we included diverse lung cancer surgeries, ranging from wedge resection to pneumonectomy. Minimally invasive surgeries are generally known to have fewer postoperative complications than open thoracotomy (7), and pneumonectomy is associated with a higher incidence of postoperative complications than other lung resection surgeries (5). Thus, our relatively more heterogenic surgical population may have impacted our results. Second, we set 30-day unplanned readmission rate, as opposed to postoperative complication, as the study endpoint; consequently, more severe postoperative complications or new complications after discharge, as opposed to mild postoperative complications that occur in the short term, may have influenced the results.
The results regarding negative FB were notable in the present study. Wu et al. (7) reported that restrictive fluid therapy during lobectomy of the lung was associated with the development of postoperative pulmonary complications. The study by Wu et al. (7) analyzed the effects of fluid administration during surgery, but our present study focused on FB status during and up to 24 hours after surgery. In addition to intraoperative fluid management, postoperative fluid management has an important influence on the prognosis of surgical patients (20). The specific conditions and intraoperative events of patients should be considered for optimal postoperative fluid management to improve postoperative outcomes. In our study, negative FB during and up to 24 hours after surgery might be controlled with close monitoring of blood pressure by the anesthesiologists and surgical teams to reduce the fluid input during the postoperative period. Therefore, patients with negative FB (<0%) might not experience the postoperative complications that could occur due to inadequate organ perfusion.
Another interesting and noteworthy aspect is that our findings are contradictory to a previous report from our institution, where 30-day unplanned readmission rate after major abdominal surgery was not associated with perioperative cumulative FB status (20). The main reason for this discrepancy is likely to be attributable to the difference in the type of surgery involved. Generally, several studies have suggested that restrictive fluid regimen has no benefit for reducing postoperative complications after abdominal surgery (21-23). Moreover, in the RELIEF trial reported by Myles et al., the liberal fluid regimen group had lower incidence of acute kidney injury after major abdominal surgery than the restrictive fluid regimen group (15). In other words, positive FB of >5% might be associated with the 30-day unplanned readmission rate in our study because we included diverse lung cancer surgeries and our study patients consisted of a surgical population for whom pulmonary complication was the most important postoperative complication (3).
In the present study, we calculated the perioperative FB status during and up to 24 hours after lung resection surgery. Myles et al. (15) also evaluated fluid management during and up to 24 hours after major abdominal surgery in the REILEF trial. Because we included diverse thoracic surgeries ranging from wedge resection to pneumonectomy in this study, the length of hospital stay of our patients varied. For example, the length of hospital stay after wedge resection is relatively short at 2–3 days. Therefore, we calculated perioperative FB during and up to 24 hours after lung resection surgery to investigate perioperative FB and 30-day unplanned readmission after lung cancer surgery.
The present study has a few limitations. First, there were some limitations due to retrospective design of the study. For example, there might be residual confounders that needed to be controlled by multivariable adjustment, and the quality and accuracy of data might be limited as compared to those of the data obtained in a prospective study. Second, our findings have limited generalizability because the study was conducted at a single center, and the results of this study might not be applicable to other institution. Third, because we could not capture data regarding readmission to other hospitals, our results might be biased. However, we used the distance from home to the hospital based on the postal code as an important covariate in the multivariable model to reduce the bias. Fourth, the relatively small sample event number (164, 6.9%) might be a concern and affect the result of our study. However, to overcome this statistical limitation, we initially screened the covariates by using a criteria of P<0.2 in the univariable logistic regression analysis, for constructing final multivariable logistic regression model. As a result, 10 variables were included in the final multivariable model, and our event per variable (EPV) was 16.2. Traditionally, an EPV >10 was recommended for multivariable logistic regression modelling to avoid overfitting (24), suggesting that our study might not have suffered from overfitting. Lastly, the sample sizes of the negative and positive FB groups were very small, which might have affected on our study. Therefore, the results should be interpreted carefully, and further study is needed with a larger sample size.
In conclusion, our findings show that perioperative positive FB (>5%) is associated with elevated 30-day unplanned readmission rate among patients who underwent lung cancer surgery. Future prospective studies are needed to confirm these findings.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1474
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1474
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1474). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study also conformed to the provisions of the Declaration of Helsinki (as revised in 2013). This retrospective observational study was approved by the institutional review board (IRB) of the Seoul National University Bundang Hospital (SNUBH) (IRB approval number: B-1903/528-101; approval date: February 25, 2019). The requirement to obtain informed consent was exempted in consideration of the retrospective design, where medical records are reviewed after completion of care.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Corcoran T, Rhodes JE, Clarke S, et al. Perioperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg 2012;114:640-51. [Crossref] [PubMed]
- Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: A prospective randomized trial. Arch Surg 2010;145:1193-200. [Crossref] [PubMed]
- Alam N, Park BJ, Wilton A, et al. Incidence and risk factors for lung injury after lung cancer resection. Ann Thorac Surg 2007;84:1085-91; discussion 1091. [Crossref] [PubMed]
- Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg 2003;97:1558-65. [Crossref] [PubMed]
- Parquin F, Marchal M, Mehiri S, et al. Post-pneumonectomy pulmonary edema: analysis and risk factors. Eur J Cardiothorac Surg 1996;10:929-32; discussion 933. [Crossref] [PubMed]
- Ashes C, Slinger P. Volume management and resuscitation in thoracic surgery. Current Anesthesiology Reports 2014;4:386-96. [Crossref]
- Wu Y, Yang R, Xu J, et al. Effects of intraoperative fluid management on postoperative outcomes after lobectomy. Ann Thorac Surg 2019;107:1663-9. [Crossref] [PubMed]
- Assaad S, Popescu W, Perrino A. Fluid management in thoracic surgery. Curr Opin Anaesthesiol 2013;26:31-9. [Crossref] [PubMed]
- Copeland LA, Graham LA, Richman JS, et al. A study to reduce readmissions after surgery in the Veterans Health Administration: design and methodology. BMC Health Serv Res 2017;17:198. [Crossref] [PubMed]
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009;360:1418-28. [Crossref] [PubMed]
- Merkow RP, Ju MH, Chung JW, et al. Underlying reasons associated with hospital readmission following surgery in the United States. JAMA 2015;313:483-95. [Crossref] [PubMed]
- Elsamadicy AA, Sergesketter A, Adogwa O, et al. Complications and 30-Day readmission rates after craniotomy/craniectomy: A single Institutional study of 243 consecutive patients. J Clin Neurosci 2018;47:178-82. [Crossref] [PubMed]
- Spataro E, Durakovic N, Kallogjeri D, et al. Complications and 30-day hospital readmission rates of patients undergoing tracheostomy: A prospective analysis. Laryngoscope 2017;127:2746-53. [Crossref] [PubMed]
- Xiao H, Quan H, Pan S, et al. Incidence, causes and risk factors for 30-day readmission after radical gastrectomy for gastric cancer: a retrospective study of 2,023 patients. Sci Rep 2018;8:10582. [Crossref] [PubMed]
- Myles PS, Bellomo R, Corcoran T, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med 2018;378:2263-74. [Crossref] [PubMed]
- Balakumar V, Murugan R, Sileanu FE, Palevsky P, Clermont G, Kellum JA. Both positive and negative fluid balance may be associated with reduced long-term survival in the critically ill. Crit Care Med 2017;45:e749-57. [Crossref] [PubMed]
- Selewski DT, Cornell TT, Lombel RM, et al. Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med 2011;37:1166-73. [Crossref] [PubMed]
- O'Connor ME, Prowle JR. Fluid Overload. Crit Care Clin 2015;31:803-21. [Crossref] [PubMed]
- Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA 2013;309:678-88. [Crossref] [PubMed]
- Oh TK, Oh AY, Hwang JW. Association between perioperative fluid balance and 30-day unplanned readmission after major abdominal surgery. Surg Innov 2019;26:401-7. [Crossref] [PubMed]
- Gómez-Izquierdo JC, Trainito A, Mirzakandov D, et al. Goal-directed fluid therapy does not reduce primary postoperative ileus after elective laparoscopic colorectal surgery: A randomized controlled trial. Anesthesiology 2017;127:36-49. [Crossref] [PubMed]
- Grant F, Brennan MF, Allen PJ, et al. Prospective randomized controlled trial of liberal Vs Restricted perioperative fluid management in patients undergoing pancreatectomy. Ann Surg 2016;264:591-8. [Crossref] [PubMed]
- Huang Y, Chua TC, Gill AJ, et al. Impact of perioperative fluid administration on early outcomes after pancreatoduodenectomy: A meta-analysis. Pancreatology. 2017;17:334-41. [Crossref] [PubMed]
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165:710-8. [Crossref] [PubMed]