Efficacy of a simple and inexpensive exercise training program for advanced chronic obstructive pulmonary disease patients in community hospitals
Introduction
Chronic obstructive pulmonary disease (COPD), a large cause of morbidity and mortality worldwide, is characterized by progressive airflow limitation that is partially reversible (1).The Global Burden of Disease Studies reported COPD as the sixth most common cause of death worldwide in 1990 and made the alarming prediction that it could rise to third by 2020 irrespective of public health intervention. Furthermore, COPD ranks as the twelfth greatest cause of chronic morbidity and is predicted to become the fourth highest disability-producing illness by 2020 (2-4). Patients with COPD are often frequent consumers of health care resources (5,6); their exacerbations and hospitalizations are often associated with a decline in lung function and increased mortality (7,8). Pulmonary rehabilitation is now an integral part of clinical management and health maintenance for those who remain symptomatic or continue to have decreasing function despite standard medical management. The principal goals of pulmonary rehabilitation include reducing symptoms, decreasing disability, increasing participation in physical and social activities and improving overall quality of life (QoL) for individuals with chronic respiratory disease (9). Exercise training is an essential part of the pulmonary rehabilitation program with varying modes of training, intensity, frequency and duration (9). The American College of Sports Medicine (ACSM) recommends that training for healthy, elderly people should include endurance, strength and flexibility (10). COPD patients have ready access to pulmonary rehabilitation in North America and parts of Europe. However, its application in developing countries like Thailand is limited (11) and might not be feasible for large scale implementation in community hospitals due to the complexity of the program and expensive training equipment, including cycle ergometry and treadmills. In this prospective study, we investigated the feasibility and long term benefits of a simple and economical exercise training program under a community hospital setting.
Methods
Subjects
Outpatients diagnosed with moderate, severe and very severe COPD (GOLD stages II-IV) (12) in a stable clinical condition from five community hospitals were prospectively enrolled from June 2006 to June 2009. The five hospitals are located in four provinces of Northern Thailand: Sanpatong and Chiang Dao Hospitals in Chiang Mai, Wieng-Pa-Pao Hospital in Chiang Rai, Pai Hospital in Mae-Hong-Son and Chiang Kam Hospital in Pha-Yao. The inclusion criteria was COPD patients diagnosed by GOLD criteria stages II-IV in a stable clinical condition aged 40 years and older, smoking history of more than 10 pack-years and a history of exacerbation at least one time in the previous year. Patients were excluded from the study if they experienced exacerbation during the previous 6 weeks before enrollment, pneumonia or a history of hospitalization during the preceding three months, long-term oxygen therapy, co-morbidities that limited their exercise tolerance, or a negative attitude toward the program. The study was approved by the Ethics Committees of the Faculty of Medicine, Chiang Mai University and filed under Thai Clinical Trials Registry (Study ID: TCTR20140926001). Written informed consent was obtained from all patients.
Study design
All COPD patients adhered to their prescribed standard medical treatment of an inhaled bronchodilator and/or corticosteroid use throughout the study period. Patients who met the criteria undertook a modified Suandok exercise training (MSET) program. The MSET program was initially developed as a mild to moderate intensity program consisting of incremental strength and endurance training performed for 35-40 minutes each session, with up to 2 sessions/week for 8 weeks. Strength training of the peripheral muscles of the upper and lower limbs was performed by weight lifting and resistive loading; and endurance training consisted of walking on a flat surface corridor. A series of strength training for upper and lower limbs was performed, preceded by a brief warm up lasting 10-15 minutes before each session. Comprehensive instructions on correct exercise technique were given and patients were supervised throughout the entire 8-week training period. Workload was fixed at 100% of the 10-repetition maximum (RM) throughout the program. During the first four weeks, patients performed three sets of strength training on each peripheral muscle group. From then on sessions were increased by ten repetitions every two weeks until a maximum of five series in the final two weeks. For endurance training, walking sessions were conducted during the first two weeks lasting 15-20 minutes at mild to moderate intensity [40-45% of heart rate reserve (HRR)] without exceeding a 6 rating of perceived exertion (RPE) (13). Exercise sessions were then increased by 5 minutes every two weeks until 35-40 minutes per session during the final week. Heart rate and finger pulse oxygen saturation (SpO2) were monitored continuously throughout the training period. A cool down period lasting 10 minutes was performed after each training session. Brief pause periods were determined according to each individual’s dyspnea level before approaching RPE =6 or SpO2 below 88%. In the maintenance phase from months 3 to 12, all patients were provided with training equipment and encouraged to continue their exercise at home without any supervision.
Outcome measures
All tests were performed by patients at the onset of the MSET program and at months 1, 2, 3, 6, 9 and 12. Post-bronchodilator spirometry parameters included forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and the ratio of FEV1 to FVC was determined by a standard spirometer (SpirobankTM, Medical International Research USA, Inc.) following American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines (13) applying reference values for Knudson 1983. Three muscle groups (biceps, triceps, and pectoralis) were selected for upper limb muscle exercise tests. Biceps and triceps strength were tested by weight load (dumbbell) and pectoralis by thera-band. The quadriceps muscle group was selected for lower limb exercises and was tested by lifting weights (sand bags). Weight lifting capacity was measured and recorded as the heaviest weight that could be lifted for a ten repetitions maximum (10-RM) (10). Exercise capacity was determined by a standard six-minute walk test and six-minute walk distance (6-MWD) was recorded (14). An increase in the 6-MWD of 54 meters was considered as the minimal clinically important difference (MCID) (15). Patients’ dyspnea levels and health-related QoL (HRQoL) were measured by using the modified Medical Research Council (mMRC) (16) and St. George’s Respiratory Questionnaire (SGRQ) (17) respectively. The MCID of mMRC and SGRQ were determined as a score decrease by at least 1 and 4 points, respectively (16,18).
Statistical analysis
Sample size calculations were based on the difference in improvement of 6-MWD between the baseline and two months after the program in our pilot study. With a mean difference of 60.4 metres and standard deviation (SD) of 80.4, a sample size of 16 subjects was needed to be able to reject the null hypothesis with probability (power) 0.8 and Type I error probability 0.05. Anticipating a drop-out rate of 30%, we therefore planned to enroll 21 patients into the study. Results for numerical values were expressed as means ± SD, range for continuous variables, and numbers (%) for categorical variables. Progress of trained muscle strength during the entire follow-up period compared to baseline was determined using unpaired t-tests with statistical significance at P<0.05. In addition MCID was determined for mMRC, 6-MWD and SGRQ. All analyses were carried out with the SPSS statistical package, version 16 for Windows.
Results
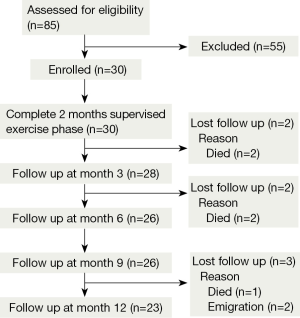
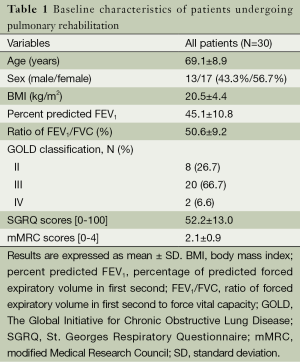
Out of 85 patients assessed for eligibility, 55 were excluded due to: history of exacerbation during the previous 6 weeks before enrollment (N=10), use of long-term oxygen therapy (N=6), and unwillingness to participate in our program (N=39). Only thirty patients met the inclusion criteria for enrollment (13 men, 17 women) with a mean age of 69.1±8.9 years, mean body mass index of 20.5±4.4 kg/m2, and mean % predicted FEV1 45.1±10.8. According to GOLD classification, 8 (26.7%) cases were in stage II, 20 (66.7%) in stage III, and 2 (6.6%) in stage IV. Additional baseline characteristics of the study patients are shown in Table 1. No significant changes in lung function were observed throughout the follow up period.
Full table
All enrolled patients completed the 8-week exercise training program. By the follow-up period, 23 had completed the full study period of 12 months. There were a total of five fatal cases during the study period; acute myocardial infarction and respiratory failure at 3 months, and COPD exacerbation at 6 (two cases) and 12 months. Two cases dropped out from the study due to emigration issues (Figure 1).
Effects of exercise training on peripheral muscle strength
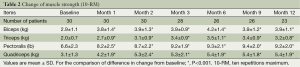
At the first month of the training period and all follow-up visits, all patients showed statistically significant strength increases in the four trained muscle groups as shown in Table 2.
Full table
Effects of exercise training on dyspnea severity, exercise capacity and quality of life
After training, there were statistically significant improvements in mMRC dyspnea level, 6-MWD, and QoL throughout the 12-month follow-up. However, the mMRC score alterations reached MCID only at months 2, 3 and 9. The changes of 6-MWD and SGRQ score reached MCID from month 2 and month 1 respectively and persisted throughout all follow up visits (Table 3).

Full table
Discussion
The results of this study indicate significant benefits of the MSET program for patients with advanced COPD. This study revealed that the MSET program was clinically effective for improving the strength of trained muscles, dyspnea levels, exercise capacity, and QoL. Very few studies have been able to demonstrate the long term benefits of exercise training (19-23). In our study the clinical parameters were maintained sufficiently well throughout one year except for dyspnea level.
The MSET program using inexpensive exercise training equipment (weight load and thera-band) included both upper and lower limb strength training as well as endurance training by walking. This strategy could be easily be integrated into an outpatient pulmonary rehabilitation program in community hospitals and is simple enough to be continued without supervision at home. The lack of a control group in the current study proved a limitation. However, the beneficial results were observed in the same trends on all clinical parameters including improvement on trained muscle strength, exercise capacity, dyspnea level (at some visits), and quality of life. Secondly, there may be a learning effect contributing to the improvement on 6-MWD because the patients in this study received only positive encouragement without any practice for walking tests before the baseline walking test (24). The observed increase was not perceived as an isolated outcome but rather correlated with improvements in muscle strength and quality of life. The authors, therefore, believe that such improvements could be attributed to adaptation to exercise rather than learning. Thirdly, our study included drop-out patients relatively lower than reported in an earlier study (20), mainly due to death from acute exacerbation and respiratory failure. Fatal patients in this study had severe disease as evidenced by low FEV1, as well as co-morbidities and frequent exacerbations as with earlier studies (25-27). However, the dropped out patients (N=7) should not have a large impact on statistical results because we had enrolled more patients (N=30) than the minimum sample size required (N=21) and used unpaired t-tests instead of repeated measures for statistical analysis. Fourthly, the heterogeneity of patients in our study could have affected individual response to the assigned exercise training protocol, but was not investigated.
High intensity whole-body exercise programs, suitable for improving fitness in healthy individuals, is usually not tolerated by COPD patients due to reduced ventilatory reserves and incapacitating breathlessness (28). In this study, after initiation of the mild to moderate intensity exercise training, significant improvements in trained muscle strength were observed from the first month and sustained throughout the remaining year. Both statistically and clinically significant improvements in QoL and exercise capacity were observed from the first and second months respectively and maintained throughout the year.
The intensity of endurance training was similar to previous studies but was performed only twice a week instead of three times a week (27,29,30). In this study, a relatively simple strength and endurance training program was shown to successfully increase the strength of all muscles that underwent training. The extent of improvement was similar to that achieved in prior studies with COPD patients (29) and elderly healthy subjects (31). Our study indicates that a combination of strength and endurance training might be the best training method for improving physical function in advanced COPD patients for a number of reasons. Firstly, most functional tasks used in routine daily activities are relatively light and brief. Stronger and longer activities that improve muscular strength are necessary (32). Secondly, previous studies in older adults have shown significant improvement of performance in daily activities following strengthening exercise (33). Exercise capacities have been shown to correlate with muscle strength independent of lung function in patients with COPD (34,35). Furthermore, weight training in patients with COPD has been reported to improve exercise performance and QoL (36,37). If muscle strength was causally responsible for the reduction in exercise capacity then its improvement should result in enhanced exercise performance as revealed in this study. However, no clinically significant differences were observed in the mMRC score measured at months 1, 6 and 12 as mMRC might not be sensitive enough to detect changes in dyspnea severity in patients with advanced COPD as reported in an earlier study (38).
ATS/ERS has recommended that exercise programs should offer at least three sessions per week to achieve physiologic benefit (9), which was later modified to twice-weekly supervised plus one unsupervised home session. Our results are contrary to that of Ringbaek et al. who did not find any improvement in COPD patients based on an exercise regime of twice a week for 8 weeks (39). The reason for these conflicting results is probably due to the differences in disease severity and training workload. The patients in this study had more severe disease [less body mass index (BMI), less FEV1, higher SGRQ], and therefore may have had more room for improvement for clinical outcomes measured after exercise training. This program might achieve higher workload because it was designed as an incremental strength and endurance exercise training with higher targeted dyspnea levels.
Our study revealed that the MSET program for advanced COPD patients receiving optimal pharmacological treatment in community hospitals was effective for improving exercise capacity and disease-specific quality of life. However adoption of the program in community hospitals should be primarily based on simplicity of the program and availability of training equipment. In addition, further analysis on cost-effectiveness of the program is required before it is widely implemented for primary care. The disease-specific outcome measures (6-MWD and SGRQ) as outlined in this study should be included in cost-effectiveness studies in addition to general health measures as the latter alone was not sensitive enough to discriminate the cost-effectiveness of the analysis (40).
Conclusions
Our findings suggest that the MSET program was effective for advanced COPD patients who are prone to limit their daily activities despite optimal pharmacological treatment. This program, with its simplicity and feasibility, can be widely used as part of a pulmonary rehabilitation program for advanced COPD patients in community hospitals.
Acknowledgements
The authors would like to convey special thanks to the five pulmonary rehabilitation alliance networks in northern Thailand (Sanpatong Hospital, Chiang Dao Hospital, Wieng-Pa-Pao Hospital, Pai Hospital and Chiang Kam Hospital) for their valuable help and support throughout the study.
C Pothirat (pulmonologist) developed the study design and carried out acquisition and interpretation of data, statistical analysis, manuscript preparation, and critical revision of intellectual contents. W Chaiwong and N Phetsuk contributed to acquisition and interpretation of data, revision of the article for important intellectual contents, and final approval of the version to be published.
Disclosure: The authors declare no conflict of interest.
References
- Celli BR, MacNee W, ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932-46. [PubMed]
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997;349:1269-76. [PubMed]
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997;349:1436-42. [PubMed]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet 1997;349:1498-504. [PubMed]
- Mapel DW, Hurley JS, Frost FJ, et al. Health care utilization in chronic obstructive pulmonary disease. A case-control study in a health maintenance organization. Arch Intern Med 2000;160:2653-8. [PubMed]
- Wouters EF. Economic analysis of the Confronting COPD survey: an overview of results. Respir Med 2003;97:S3-14. [PubMed]
- Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847-52. [PubMed]
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60:925-31. [PubMed]
- Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13-64. [PubMed]
- Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334-59. [PubMed]
- Pothirat C, Phetsuk N, Deesomchok A, et al. Clinical characteristics, management in real world practice and long-term survival among COPD patients of Northern Thailand COPD club members. J Med Assoc Thai 2007;90:653-62. [PubMed]
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532-55. [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. [PubMed]
- Redelmeier DA, Bayoumi AM, Goldstein RS, et al. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med 1997;155:1278-82. [PubMed]
- Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581-6. [PubMed]
- Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respir Med 1991;85 Suppl B:25-31; discussion 33-7.
- Schünemann HJ, Griffith L, Jaeschke R, et al. Evaluation of the minimal important difference for the feeling thermometer and the St. George's Respiratory Questionnaire in patients with chronic airflow obstruction. J Clin Epidemiol 2003;56:1170-6. [PubMed]
- Güell R, Casan P, Belda J, et al. Long-term effects of outpatient rehabilitation of COPD: A randomized trial. Chest 2000;117:976-83. [PubMed]
- Troosters T, Gosselink R, Decramer M. Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Am J Med 2000;109:207-12. [PubMed]
- Ries AL, Kaplan RM, Myers R, et al. Maintenance after pulmonary rehabilitation in chronic lung disease: a randomized trial. Am J Respir Crit Care Med 2003;167:880-8. [PubMed]
- Swerts PM, Kretzers LM, Terpstra-Lindeman E, et al. Exercise reconditioning in the rehabilitation of patients with chronic obstructive pulmonary disease: a short- and long-term analysis. Arch Phys Med Rehabil 1990;71:570-3. [PubMed]
- Strijbos JH, Postma DS, van Altena R, et al. A comparison between an outpatient hospital-based pulmonary rehabilitation program and a home-care pulmonary rehabilitation program in patients with COPD. A follow-up of 18 months. Chest 1996;109:366-72. [PubMed]
- Guyatt GH, Pugsley SO, Sullivan MJ, et al. Effect of encouragement on walking test performance. Thorax 1984;39:818-22. [PubMed]
- Gerardi DA, Lovett L, Benoit-Connors ML, et al. Variables related to increased mortality following out-patient pulmonary rehabilitation. Eur Respir J 1996;9:431-5. [PubMed]
- Fischer MJ, Scharloo M, Abbink JJ, et al. Drop-out and attendance in pulmonary rehabilitation: the role of clinical and psychosocial variables. Respir Med 2009;103:1564-71. [PubMed]
- Spruit MA, Gosselink R, Troosters T, et al. Resistance versus endurance training in patients with COPD and peripheral muscle weakness. Eur Respir J 2002;19:1072-8. [PubMed]
- Vogiatzis I, Zakynthinos S. Factors limiting exercise tolerance in chronic lung diseases. Compr Physiol 2012;2:1779-817. [PubMed]
- Bernard S, Whittom F, Leblanc P, et al. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159:896-901. [PubMed]
- Ortega F, Toral J, Cejudo P, et al. Comparison of effects of strength and endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:669-74. [PubMed]
- Frontera WR, Meredith CN, O'Reilly KP, et al. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol (1985) 1988;64:1038-44. [PubMed]
- Rantanen T, Era P, Heikkinen E. Maximal isometric knee extension strength and stair-mounting ability in 75- and 80-year-old men and women. Scand J Rehabil Med 1996;28:89-93. [PubMed]
- Skelton DA, Young A, Greig CA, et al. Effects of resistance training on strength, power, and selected functional abilities of women aged 75 and older. J Am Geriatr Soc 1995;43:1081-7. [PubMed]
- Bernard S, LeBlanc P, Whittom F, et al. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;158:629-34. [PubMed]
- Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med 1996;153:976-80. [PubMed]
- Simpson K, Killian K, McCartney N, et al. Randomised controlled trial of weightlifting exercise in patients with chronic airflow limitation. Thorax 1992;47:70-5. [PubMed]
- Clark CJ, Cochrane LM, Mackay E, et al. Skeletal muscle strength and endurance in patients with mild COPD and the effects of weight training. Eur Respir J 2000;15:92-7. [PubMed]
- Evans RA, Singh SJ, Collier R, et al. Pulmonary rehabilitation is successful for COPD irrespective of MRC dyspnoea grade. Respir Med 2009;103:1070-5. [PubMed]
- Ringbaek TJ, Broendum E, Hemmingsen L, et al. Rehabilitation of patients with chronic obstructive pulmonary disease. Exercise twice a week is not sufficient! Respir Med 2000;94:150-4. [PubMed]
- Gillespie P, O'Shea E, Casey D, et al. The cost-effectiveness of a structured education pulmonary rehabilitation programme for chronic obstructive pulmonary disease in primary care: the PRINCE cluster randomised trial. BMJ Open 2013;3:e003479. [PubMed]