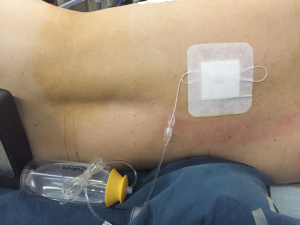
Paravertebral block with modified catheter under surgeon’s direct vision after video-assisted thoracoscopic lobectomy
Introduction
Video-assisted thoracoscopic surgery (VATS) was introduced to replace open thoracic surgery in high-volume experienced centers due to its several superiorities, including fewer postoperative complications, better preservation of respiratory function and shorter postoperative hospital stay (1-3). However, the moderate-to-severe postoperative pain was found in patients who benefit from VATS on account of the ports and chest drains, and the extent of surgery (4). Therefore, analgesia remains an important consideration after VATS lobectomy.
Numerous pain management options for VATS have been reported in the recent studies, including non-steroidal anti-inflammatory drugs (NSAIDs), thoracic epidural analgesia (TEA), systemic opioids and paravertebral block (PVB) (5-7). Over the years, TEA has been considered the golden standard of analgesia for thoracic surgery (8). Recently, multiple meta-analyses revealed that PVB can provide comparable pain relief to TEA with less frequent adverse reactions after thoracic surgery (9,10). Besides, continuous paravertebral block was indicated as equally effective as epidural block for pain management after VATS lobectomy, and might have a better profile of safety than epidural block (11). However, the procedure and device of PVB for thoracoscopic surgery has not been well established (12). Despite the methods of ultrasound-guide that can be applied, PVB using the classical landmark puncture technique is still considered not satisfactorily predictable (13-15). Recently, the method of catheter insertion into the paravertebral space which could be verified continuously by the surgeon using the camera is preferable in some centers (11,16). Furthermore, no gold standard for PVB device has been introduced for patients undergoing VATS so far, especially for the paravertebral catheter. Currently, the conventional epidural catheter was still applied in PVB procedure in several researches.
A modified PVB (MPVB) catheter which was placed in the paravertebral space under thoracoscopic guidance by the surgeons has been employed for patients undergoing VATS lobectomy in our institution since 2017. The aim of this study was to explore whether the modified PVB catheter has any advantages over the conventional epidural catheter used for PVB in pain management after VATS lobectomy. We present the following article in accordance with the STROBE Guideline (available at http://dx.doi.org/10.21037/jtd-20-1068B).
Methods
Patients
In order to minimize selection bias, we retrospectively reviewed the database of consecutive 356 patients who underwent VATS lobectomy and extended lymphadenectomy with PVB by a single surgical team (YS) from July 2015 to December 2018 at our department. All patients were evaluated suitable for VATS preoperatively and no intraoperative conversion to open thoracotomy was happened in these 356 patients. The exclusion criteria were listed as followings: the American Society of Anesthesiologists (ASA) physical status classification greater than 3; chronic analgesic use or preexisting chronic pain syndromes; presence of chest surgery history or kyphoscoliosis; and patient records lacked sufficient information for analysis. Before March 2017, PVB was conducted with the conventional epidural catheter at our department. After that, the modified paravertebral catheter was applied for PVB in patients undergoing VATS lobectomy. The 356 patients were divided into two groups according to the catheter applied in PVB procedure (PVB group and MPVB group). Both groups had the same management and patient selection protocols. Uniportal VATS was tried to accomplish all of the operation first. If there was severe adhesion in the chest cavity or the operation from one port was hard, multi ports would be added to assist in completing the operation. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. This study was reviewed and approved by the Institutional Review Board of the Jingling Hospital (approval number 2015NZKY-028-03). All patients enrolled completed the informed consent form. Age, gender, ASA classification, type of VATS (multi-portal or uni-portal VATS), number of harvested lymph nodes and TNM stage were recorded. Patient were instructed in how to assess their pain using a visual analog scale (VAS) of 0–10 cm (0 cm: no pain, 10 cm: worst pain imaginable) after surgery.
Procedure of anesthesia and VATS lobectomy
Noninvasive blood pressure (NIBP), electrocardiography, and pulse oximetry were monitored, after which an intravenous saline infusion (10 mL/kg/h) was started. Meanwhile, general anesthesia and resuscitation were kept ready. General anesthesia was induced with 2.0 mg/kg of propofol, 2.0 µg/kg of fentanyl, and 1.0 mg/kg of rocuronium. A double-lumen endotracheal tube was placed and appropriate position confirmed. Anesthesia was maintained with continuous infusion of propofol at the rate of 8–12 mg/kg/h, with 2 µg/kg of fentanyl and 0.15 mg/kg of rocuronium bolused every 30 minutes. Then, surgery was performed with one-lung ventilation. Lobectomy resection with radical lymphadenectomy were performed in all the patients in this study.
Technique of postoperative analgesia
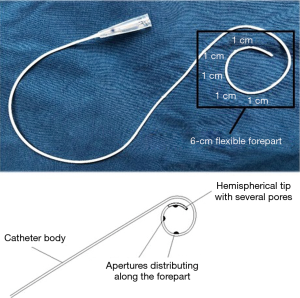
In the two groups, conventional epidural catheter and modified paravertebral catheter were both placed under sterile conditions upon completion of surgery. The percutaneous puncture point, which is equidistant to the upper and lower intercostal space, was marked 2–3 cm lateral to the midline. A 22-gauge Tuohy needle was advanced perpendicularly through the chest wall until the needle tip was visible in both groups. Then a conventional epidural catheter (B/Braun, Melsungen, Germany) was passed through the needle into the paravertebral space in the PVB group, while a modified paravertebral catheter (Fullcare, Beijing, China) was applied in the MPVB group. The tip of the modified paravertebral catheter was hemispherical with several pores and the 6 cm flexible forepart of the modified catheter had more apertures distributing tightly than the conventional epidural catheter (Figure 1). After injection of 20 mL saline created an extrapleural detachment pocket (Video 1), the modified catheter was inserted approximately 6 cm into the paravertebral space under direct visual control by the surgeon who confirmed its correct location. If pleural perforation or hematoma under parietal pleura happened during the process, another alternative insertion point would be selected. After catheter placement, an initial dose of 0.3 mL/kg of 0.2% ropivacaine, 0.05% bupivacaine and 0.26% lidocaine hydrochloride were infused into the paravertebral space through the catheter. Then an 150 mL infusion pump containing of the same ropivacaine, bupivacaine and lidocaine hydrochloride mixture was connected to the catheter to provide a continuous infusion after surgery (Figure 2). The infusion rate of the mixture into the paravertebral space could be approximately 2 mL/h if the channel was absolutely unobstructed.
Postoperative care
Patients were transferred to the intensive care unit of our department after replacing the double lumen tube to single lumen tube and extubated after completely waking up. If patients reported a VAS score >4 postoperatively, intramuscular dezocine 10 mg (Jiangsu, China) was administered as a rescue medication. The chest tube was removed when there was no leakage and the volume of drainage was less than 200 mL/24 hours, while the paravertebral catheter was also removed. The paravertebral catheter would be removed on post-operative day (POD) 3 with drainage required for more than three days after surgery. After removing chest drainage tubes, pain management was remained with 500 mg paracetamol oral route every 6 hours if VAS score over 4. The criteria for hospital discharge included pain controlled by oral analgesics, chest tube removal and assessment of patients’ well-being by attending doctors.
Clinical outcomes
The following outcomes were assessed: (I) the effect of postoperative pain management including VAS scores at the 2nd, 6th, 12th, 24th and 48th postoperative hours (at the state of rest/coughing) and the number of patients who required rescue medication before removing the pump; (II) respiratory function including spirometry values and blood gas analysis on postoperative days (PODs); (III) adverse events related to the analgesia, including respiratory depression (respiratory rate <8 breaths/min), nausea, vomiting, urinary retention, hypotension and headache; (IV) characteristics of PVB procedure, including pleural perforation and hematoma under parietal pleura as well as the consumption of drugs in the PVB pump; and (V) postoperative data including chest tube indwelling time, length of postoperative hospital stay and major complications. Hypotension was defined as systolic arterial pressure below 90 mmHg and/or systolic arterial pressure decrease by >20% compared with the pre-surgical value.
Statistical analysis
IBM SPSS for Windows, Version 22.0 (IBM Corporation, Armonk, NY, USA) was used for all statistical evaluations. Continuous variables were expressed as mean ± SD and were compared using independent samples Student’s t-test or Mann-Whitney U test. Categorical variables were summarized as proportions or percentages and were compared using χ2 test. P values less than 0.05 were considered statistically significant.
Results
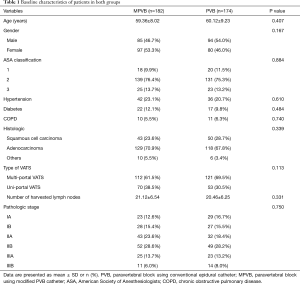
There were 172 patients who received PVB with conventional epidural catheter (PVB group), and the other 184 patients were performed PVB with modified paravertebral catheter (MPVB group). The clinical characteristics of the PVB group and MPVB group are presented in Table 1. There were no significant differences between the two groups in terms of gender, age, ASA classification, preoperative comorbidities and pathologic stage (P>0.05).
Full table
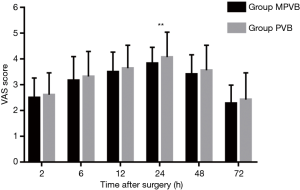
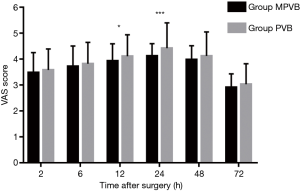
Postoperative pain scores at rest were compared at 2, 6, 12, 24, 48 and 72 hours. Significant lower pain score was found in MPVB group than that in PVB group at 24 h postoperatively (P=0.006; Figure 3). Pain scores on coughing were also compared at 2, 6, 12, 24, 48 and 72 hours. The pain scores in MPVB group were significantly lower than that in PVB group at 12 and 24 h postoperatively (P=0.037 and P<0.001, respectively; Figure 4). The number of patients who need for rescue medication via intramuscular dezocine before the infusion pump removed was significantly lower in MPVB group (10 in MPVB group versus 21 in PVB group) (P=0.028).
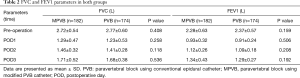
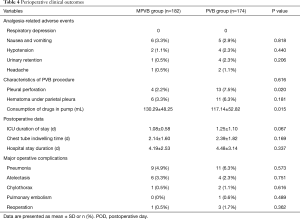
There was no significant difference of postoperative FVC and FEV1 between the two groups (Table 2). Arterial blood gas analysis demonstrated normal range of both pH and PaCO2 levels in two groups on PODs (Table 3). As shown in Table 4, no patients in either group suffered respiratory depression. There was no significant difference in other analgesia-related adverse events such as nausea and vomiting, hypotension, urinary retention and headache between the two groups. Moreover, the incidence of major complications—i.e., pneumonia, atelectasis, chylothorax, pulmonary embolism and reoperation—were comparable in both groups. In terms of the data related to PVB procedure, pleural perforation was observed in four and thirteen patients in the MPVB and PVB groups, respectively (2.2% vs. 7.5%, P=0.020), and hematoma under the parietal pleura occurred in six and eleven patients in the MPVB and PVB groups, respectively (3.3% vs. 6.3%, P=0.181). Upon the time of infusion pump removed, the consumption of analgesic drugs was significant lower in the PVB group (P=0.015). No cases of 30-day mortality were identified in all patients.
Full table

Full table
Full table
Discussion
In our study, we aimed to assess the efficacy and safety of paravertebral block with a modified catheter under surgeon’s direct vision after VATS lobectomy. VATS lobectomy was first introduced in 1992 (17), since which it has been increasingly performed as an alternative to thoracotomy due to the minimally invasive nature of the procedure and its many superiorities (2). However, acute pain after VATS could still be severe, with potential to evolve into chronic pain (16).
Continuous epidural thoracic anesthesia was considered as the gold standard of analgesia after thoracotomy and also routinely used after VATS in some centers (18,19), but the results of meta-analyze and other studies have demonstrated that paravertebral block can substantially enhance pain management for thoracoscopic surgery and have a better profile of safety than epidural block, which is particularly visible in the lower incidences of hypotension and urinary retention (11,20). Paravertebral block, introduced by Eason et al. (21), is a regional block technique. In recent years, it has been gradually developing as an alternative pain management after surgeries. Paravertebral blocks are implemented with several approaches, varying from a percutaneous needle below or above the transverse vertebral process for single shot or prolonged catheter infusion to visually guided placement of a catheter during surgery (22). Currently, there is no clear united standard for procedures of PVB in patients undergoing VATS. Some authors believe that paravertebral block using the classical landmark puncture technique might be controlled in single injection technique, but was unstable for the continuous block (13). Despite the methods of identification by ultrasound-guide, the final location of paravertebral catheters is still the major issue (14,15). In a number of centers, advancement of the needle and insertion of the catheter were verified continuously by the surgeon using the camera (16,23), and this measure was also performed in this study. Though some authors believed that start PVB before skin incision tended to show better pain control in thoracotomy (24), we supposed that start PVB upon completion of the VATS could achieve similar effect because the operation of VATS was minimally invasive, and this measure has been reported in several studies (16,23,25). Furthermore, the device which could be specifically used for PVB after VATS has not been reported so far, and the device used in other studies is conflicting, especially for the paravertebral catheter. Wu et al. (23) reported new forceps used for catheterization and the conventional epidural catheter (19G; Pajunk GmbH Medizintechnologie, Germany) was placed in the paravertebral space as the paravertebral catheter. Komatsu et al. (26) also used a commercially available 18-gauge epidural catheter as indwelling extrapleural catheter, while 82 of the 278 patients (29.5%) met pleural disruption during PVB catheterization in their report. In this study, though the similar Tuohy needle was used in both groups, the modified catheter applied in MPVB group had a flexible forepart and several pores were distributed on the hemispherical tip of the catheter, which may contribute to the lower incidence of pleural perforation in the MPVB group than that in the PVB group.
The use of a PVB catheter placed by the surgeons for continuous infusion of local analgesia was demonstrated to be as effective as the epidural block for thoracic surgery (27,28). However, the effect of continuous analgesia of PVB is related to the location of paravertebral catheter and the diffusion of analgesic drugs among the paravertebral space. Theoretically, the paravertebral pump in this study could provide approximately 2 mL/h infusion of drugs to the paravertebral space if the channel was unobstructed. However, we found substantial analgesia residue left when the pumps were removed in patients undergoing PVB with conventional epidural catheter, which may reflect inadequate local analgesic. After application of the modified catheter, the consumption of mixture in the pump of MPVB group was larger than that in the pump of PVB group, which might show a better infusion of drags through the modified catheter. The modified paravertebral catheter had 6-cm flexible forepart coiled beneath the pleura and more apertures were distributed tightly along the forepart than the conventional epidural catheter, which may contribute to the diffusion of drugs into the paravertebral space in the MPVB group. As shown in other researches, larger number of dermatome inhibition could increase more analgesia efficacy (16). In this study, the mean VAS score at 12 h after surgery during coughing states was significant lower in the MPVB group than that in the PVB group, and the mean VAS score at 24 h after surgery during rest or coughing states were both lower in the MPVB group than those in the PVB group. Furthermore, the number of patients who need for rescue medication before the pump removed was also significantly lower in MPVB group, which also revealed a better pain management effect of PVB with modified catheter. However, if drugs from the pump were too much, it may enter epidural space and increase the risk of adverse reactions. Giang et al. (16) reported a patient-controlled analgesia device (Perfusor Space; B/Braun) which was programmed to provide a continuous infusion at 3 mL/h and to permit a 2 mL of demand bolus with a 10-minute lockout interval, limited to 25 mL over 4 hours. We supposed that the patient-controlled analgesia device could be added to the modified catheter in our further investigation.
The thoracic epidural block was also routinely used after VATS, but several studies demonstrated that patients who applied epidural block may accomplished with more side effects than the patients who applied paravertebral block after VATS (11). In this study, no patients in either group suffered respiratory depression. Though a few of patients in both groups suffered other analgesia-related adverse events, the incidence was low and there was no significant difference between the two groups. What’s more, thoracic surgery has the potential to severely compromise respiratory mechanics and gas exchange, which may delay the recovery of patients and result in prolonged postoperative hospital stay (29,30). In this study, we assessed spirometry results and arterial blood gas analysis to evaluate pulmonary function. In both groups, FVC and FEV1 had decreased significantly on POD1 compared to baseline values and recovered from POD1 to POD3. There was no significant difference of FVC and FEV1 between the two groups on POD1-3. Arterial blood gas analysis showed normal ranges of both pH and PaCO2 in two groups after surgery. PaO2 may have lower reference value because it depends on oxygen support to some degree. In this study, the incidence of pulmonary complications including pneumonia and atelectasis were comparable between the two groups, and there is no significant difference between the two groups in terms of other major complications such as chylothorax, pulmonary embolism and reoperation.
Recently, the serratus plane block and other regional blocks have been widely applied in VATS. Wang et al. (31) performed serratus plane block and thoracic paravertebral block on patients after uniportal video-assisted thoracoscopic surgery and they demonstrated comparable postoperative pain management effect between the two approaches. However, PVB with modified catheter performed in this study was conducted upon the completion of surgery, and its pain management effect compared with other regional blocks such as serratus plane block or epidural block remains to be further explored.
Conclusions
PVB with the modified catheter under surgeon’s direct vision could provide better regional analgesic effect, comparable respiratory function and analgesia-related adverse events, and lower incidence of pleural perforation than PVB with the conventional epidural catheter. We demonstrated that PVB with the modified catheter under surgeon’s direct vision might be considered as a safe and effective approach for pain management after video-assisted thoracoscopic lobectomy.
Limitation
The VAS scores were evaluated in our study. However, patients’ satisfaction and life quality were not mentioned, which could cause insufficient evidence to reflect patients’ subjective feeling. Meanwhile, we are preparing for a new randomized controlled trial (RCT) to compare the safety and efficacy between modified catheter and conventional epidural catheter for PVB.
Acknowledgments
We thank all the members of department of cardiothoracic surgery in our hospital that participated in this research. We also thank XiaoKun Li for his advice on statistical analysis.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81172032) and the Natural Science Foundation of Jiangsu Province (BK20181239).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1068B
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1068B
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-1068B
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1068B). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. This study was reviewed and approved by the Institutional Review Board of the Jingling Hospital (approval number 2015NZKY-028-03). All patients enrolled completed the informed consent form. The study outcomes will not affect the future patient management. This study is based on data retrieved from a hospital medical record system. All personal data have been protected and secured according to current national and international laws.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grogan EL, Jones DR. VATS lobectomy is better than open thoracotomy: what is the evidence for short-term outcomes? Thorac Surg Clin 2008;18:249-58. [Crossref] [PubMed]
- Whitson BA, Groth SS, Maddaus MA. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8.
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Steegers MA, Snik DM, Verhagen AF, et al. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain 2008;9:955-61. [Crossref] [PubMed]
- Kim JA, Kim TH, Yang M, et al. Is intravenous patient controlled analgesia enough for pain control in patients who underwent thoracoscopy? J Korean Med Sci 2009;24:930-5. [Crossref] [PubMed]
- Steinthorsdottir KJ, Wildgaard L, Hansen HJ, et al. Regional analgesia for video-assisted thoracic surgery: a systematic review. Eur J Cardiothorac Surg 2014;45:959-66. [Crossref] [PubMed]
- Kaplowitz J, Papadakos PJ. Acute pain management for video-assisted thoracoscopic surgery: an update. J Cardiothorac Vasc Anesth 2012;26:312-21. [Crossref] [PubMed]
- El-Tahan MR. Role of Thoracic Epidural Analgesia for Thoracic Surgery and Its Perioperative Effects. J Cardiothorac Vasc Anesth 2017;31:1417-26. [Crossref] [PubMed]
- Ding X, Jin S, Niu X, et al. A comparison of the analgesia efficacy and side effects of paravertebral compared with epidural blockade for thoracotomy: an updated meta-analysis. PLoS One 2014;9:e96233. [Crossref] [PubMed]
- Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy--a systematic review and meta-analysis of randomized trials. Br J Anaesth 2006;96:418-26. [Crossref] [PubMed]
- Kosiński S, Fryźlewicz E, Wiłkojć M, et al. Comparison of continuous epidural block and continuous paravertebral block in postoperative analgaesia after video-assisted thoracoscopic surgery lobectomy a randomised, non-inferiority trial. Anaesthesiol Intensive Ther 2016;48:280-7. [PubMed]
- Daly DJ, Myles PS. Update on the role of paravertebral blocks for thoracic surgery: are they worth it? Curr Opin Anaesthesiol 2009;22:38-43. [Crossref] [PubMed]
- Luyet C, Siegenthaler A, Szucs-Farkas Z, et al. The location of paravertebral catheters placed using the landmark technique. Anaesthesia 2012;67:1321-6. [Crossref] [PubMed]
- Marhofer P, Kettner SC, Hajbok L, et al. Lateral ultrasound-guided paravertebral blockade: an anatomical-based description of a new technique. Br J Anaesth 2010;105:526-32. [Crossref] [PubMed]
- Cowie B, McGlade D, Ivanusic J, et al. Ultrasound-guided thoracic paravertebral blockade: a cadaveric study. Anesth Analg 2010;110:1735-9. [Crossref] [PubMed]
- Giang NT, Van Nam N, Trung NN, et al. Patient-controlled paravertebral analgesia for video-assisted thoracoscopic surgery lobectomy. Local Reg Anesth 2018;11:115-21. [Crossref] [PubMed]
- Roviaro G, Rebuffat C, Mariani C, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Yie JC, Yang JT, Wu CY, et al. Patient-controlled analgesia (PCA) following video-assisted thoracoscopic lobectomy: comparison of epidural PCA and intravenous PCA. Acta Anaesthesiol Taiwan 2012;50:92-5. [Crossref] [PubMed]
- Yoshioka M, Mori T, Kobayashi H, et al. The efficacy of epidural analgesia after video-assisted thoracoscopic surgery: a randomized control study. Ann Thorac Cardiovasc Surg 2006;12:313-8. [PubMed]
- Hu Z, Liu D, Wang ZZ, et al. The efficacy of thoracic paravertebral block for thoracoscopic surgery: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2018;97:e13771. [Crossref] [PubMed]
- Eason MJ, Wyatt R. Paravertebral thoracic block-a reappraisal. Anaesthesia 1979;34:638-42. [Crossref] [PubMed]
- Norum HM, Breivik H. A systematic review of comparative studies indicates that paravertebral block is neither superior nor safer than epidural analgesia for pain after thoracotomy. Scand J Pain 2010;1:12-23. [Crossref] [PubMed]
- Wu Z, Fang S, Wang Q, et al. Patient-Controlled Paravertebral Block for Video-Assisted Thoracic Surgery: A Randomized Trial. Ann Thorac Surg 2018;106:888-94. [Crossref] [PubMed]
- Yamauchi Y, Isaka M, Ando K, et al. Continuous paravertebral block using a thoracoscopic catheter-insertion technique for postoperative pain after thoracotomy: a retrospective case-control study. J Cardiothorac Surg 2017;12:5. [Crossref] [PubMed]
- Kadomatsu Y, Mori S, Uchiyama M, et al. Comparison of the analgesic effects of modified continuous intercostal block and paravertebral block under surgeon's direct vision after video-assisted thoracic surgery: a randomized clinical trial. Gen Thorac Cardiovasc Surg 2018;66:425-31. [Crossref] [PubMed]
- Komatsu T, Sowa T, Kino A, et al. The importance of pleural integrity for effective and safe thoracic paravertebral block: a retrospective comparative study on postoperative pain control by paravertebral block. Interact Cardiovasc Thorac Surg 2015;20:296-9. [Crossref] [PubMed]
- Elsayed H, McKevith J, McShane J, et al. Thoracic epidural or paravertebral catheter for analgesia after lung resection: is the outcome different? J Cardiothorac Vasc Anesth 2012;26:78-82. [Crossref] [PubMed]
- Scarci M, Joshi A, Attia R. In patients undergoing thoracic surgery is paravertebral block as effective as epidural analgesia for pain management? Interact Cardiovasc Thorac Surg 2010;10:92-6. [Crossref] [PubMed]
- Muehling BM, Halter GL, Schelzig H, et al. Reduction of postoperative pulmonary complications after lung surgery using a fast track clinical pathway. Eur J Cardiothorac Surg 2008;34:174-80. [Crossref] [PubMed]
- DO W. Preventing postoperative pulmonary complications: the role of the anesthesiologist. Anesthesiology 2000;92:1467-72. [Crossref] [PubMed]
- Wang L, Wang Y, Zhang X, et al. Serratus anterior plane block or thoracic paravertebral block for postoperative pain treatment after uniportal video-assisted thoracoscopic surgery: a retrospective propensity-matched study. J Pain Res 2019;12:2231-8. [Crossref] [PubMed]