
Determining the optimal puncture site of CT-guided transthoracic needle aspiration biopsy for the diagnosis of tuberculosis
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis complex (MTBC), remains one of the deadliest infectious diseases worldwide (1). According to the estimation by World Health Organization (WHO), approximately 10.0 million new cases of TB occurred, and 1.6 million people died from this disease globally in 2017 (2). China has the world’s third largest TB epidemic, accounting for approximate 9% of global TB burden (2). Despite the great effort over decades in fighting against TB, the emergence of multidrug-resistant TB poses a major challenge for future TB control in this country (3).
Diagnosis of TB usually relies on clinical presentation, radiological findings and laboratory examinations (4). Detection of MTB by smear microscopy and mycobacterial culture has stagnated at around 50% of the total of new cases (5) which means that nearly half of TB patients are diagnosed without the positive bacterial results from laboratory examination. As an alternative, diagnosis decisions have to rely on other laboratory-based indicators, such as tuberculin skin test and blood-based interferon-γ release assay (6). Unfortunately, all these latter methods are non-specific for active TB patients, which inevitably results in the misdiagnosis of TB among individuals with suspected TB (6). In view of the foregoing, there is an urgent need to develop more feasible methods aiming at detecting MTB in clinical samples from the TB patients without laboratory bacteria evidence.
Computed tomography (CT)-guided biopsy is a well-established technique for the diagnosis of pulmonary lesions (7,8). Although this technique has been widely used for identifying patients with known or suspected lung cancer (8), the usefulness of technique for patients with suspected TB is poorly understood due to its invasive measures. In addition, the fact that multiple ill-defined nodules observed in the lungs of TB patients is considered as another challenge for clinicians to determine the optimal puncture site among heterogeneous radiologic manifestations (9). Thus, the purpose of this study was to retrospectively assess the diagnostic yield of CT-guided transthoracic needle aspiration biopsy for the diagnosis of TB without laboratory bacteria evidence. We also aimed to determine the optimal puncture site by the comparison of radiological characteristics of TB patients stratified to different histopathological results.
Methods
Patients
We retrospectively analysed the data of clinically diagnosed TB patients with negative smear microscopy, mycobacterial culture and GeneXpert results between July 2016 and June 2018. The clinically diagnosed TB patients met the following criteria: (I) clinical TB symptoms; (II) clinical-radiologic picture highly suggestive of TB; (III) satisfactory response to anti-TB treatment (10). Prior to lung biopsy, all lesions were scanned using routine CT performed at a thickness of 5.0 mm. The demographic information was retrieved from the clinical chart records of patients, including age gender, smoking status, comorbidity, laboratory examinations, and response to treatment. None of these patients were on anti-TB treatment before lung biopsy.
CT acquisition
All CT scans were carried out with a 64-multidetector GE Healthcare CT860 HD scanner (GE Healthcare, Milwaukee, Wisconsin). The parameters used were: 250 mA; 120 kV; and inspiratory volumetric acquisition from lung apex to base with 5 mm contiguous slices. The images were obtained with lung window (window width: 1,500 HU; window level, −500 HU), and mediastinal window (window width, 350 HU, window level, 40 HU).
For the targeted lesion, a contrast-enhanced CT scan of the chest was performed as previously described. Images were obtained with a lung window (window width: 1,500 HU; window level, −500 HU), and mediastinal window (window width: 350 HU, window level, 40 HU) when the patient was placed in the supine position at full end-inspiration status. The scans were acquired from 5 mm above target lesion to 5 mm below targeted lesion with a slice thickness of 2.5 mm. All images were reviewed on picture archiving and communication system by two radiologists with ≥5 years of image interpretation experience. The discordant results were rechecked by the third experienced radiologist. The maximum diameters of lesions were measured on lung window; and the densities in lesions were calculated as the mean value of three measured densities on mediastinal window.
CT interpretation
The targeted lesions were divided into 4 categories on the basis of CT images: consolidation (defined as dense opacification without forming mass), nodule (round shaped opacity ≤3 cm in diameter, regardless of margin), mass (>3 cm in diameter) and cavity (presence of air within pulmonary consolidation, a mass, or a nodule) (11).
Biopsy procedures
All biopsy procedures were performed under conventional CT guidance by an experienced radiologist as previously reported (8,12). Briefly, the patients were placed in the prone, supine or lateral position depending on the location of lesion. After the administration of a local anesthetic, all biopsies were conducted with a coaxial needle system consisting of a 17-gauge coaxial introducer needle (Argon Medical Devices, Plano, Texas), and an 18-gauge automated cutting biopsy needle (Argon Medical Devices, Plano, Texas). The introducer needle was firstly positioned during a single breath-hold into the proximal aspect of the lesion. Then the biopsy needle was inserted coaxially into the guiding canula to obtain the fresh tissue specimen. In addition, specimens were immediately fixed by immersion in 10% formalin solution and were sent to the pathologist for examination.
Histopathology examinations
The formalin-fixed specimens were embedded into a paraffin block for pathological diagnostics. The block was firstly trimmed at 15–30 µm, followed by preparation of 4 µm of ribbons for staining. One to four ribbons were strained with hematoxylin and eosin; the second one to four ribbons were obtained for Ziehl-Neelsen (Z-N) staining. In addition, ten ribbons were transferred in 1.5 mL Eppendorf tube for DNA extraction according the manufacturer’s instructions (13). The crude genomic DNA was used as template for real-time PCR analysis with commercial kit (TB-DNA, DAAN Gene, Guangzhou, China). The real-time PCR cut‐off value was set at a cycle threshold (Ct) value of 35.
Statistical analysis
Statistical analysis performed with logistic regression analyses confirmed the factors contributing to the positive results by laboratory method. In addition, patients were stratified using the results of CT-guided transthoracic needle aspiration biopsy. Medians of value of adding unenhanced CT to contrast-enhanced CT (ΔCT density) were compared using Wilcoxon rank-sum tests. We also used receiver operating characteristic (ROC) curve analysis to determine the cut-off value of ΔCT density producing the most potent performance for the detection of MTB from biopsy tissue. The difference was declared as significant if P value was less than 0.05.
This study was approved by the Ethics Committee of Beijing Chest Hospital, Capital Medical University (YJS-2019008). All these patients experienced CT-guided lung biopsied after the acquisition of written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Results
Diagnostic accuracy
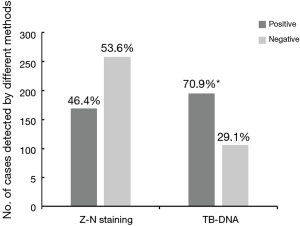
We retrospectively analysed 356 TB patients who underwent CT-guided lung biopsies between July 2016 and June 2018. Of these 356 patients, 258 (72.5%) were male. None of them were seropositive for HIV, and the most frequent comorbidity was diabetes (39/256, 11.0%). In addition, all patients had no previous anti-TB treatment history. Based on examinations with lung biopsies, 169 had positive results by Z-N staining, yielding a positive rate of 46.4% (169/356). For TB-DNA, the additional 89 patients were identified as TB cases, demonstrating an overall positive rate of 70.9% (258/356). Statistical analysis revealed that the positive rate of TB-DNA was significantly higher than that of Z-N staining (P<0.001) (Figure 1).
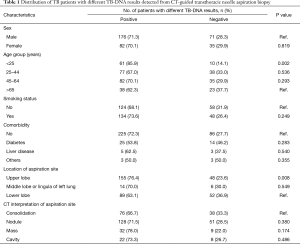
The results of the univariate analysis to identify risk factors for negative diagnostic results are shown in Table 1. Patients aged <25 years (85.9%, 61/71) were more likely to have positive histologic results compared with those aged >65 years (62.3%, 38/61, P=0.002). In addition, the location of aspiration site seemed to be associated with the detection results. As summarized in Table 1, the positive rate of lesions from upper lobe (76.4%, 155/203) was significantly higher than that from lower lobe (63.1%, 89/141, P=0.008). In contrast, sex, comorbidity, smoking status and the CT interpretation of aspiration site had no effect on the positive rate of histologic results (P>0.05).
Full table
Correlation between enhancement of CT density and histologic finding
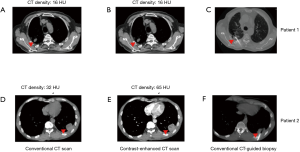
We further analysed the correlation between enhancement of CT density and histologic finding. Two representative CT-guided lung biopsy cases are shown in Figure 2. For Patient 1, the conventional and contrast-enhanced CT densities of lesion were both 14 HU. In contrast, the CT density of lesion from Patient 2 was increased from 36 to 65 HU for contrast-enhanced evaluation.
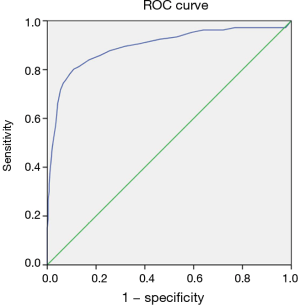
To identify the cut-off value of ΔCT density, the patients were divided into a positive histological group and a negative histological group according to the histologic findings. The mean of ΔCT density were 12.84±6.81 and 28.32±9.82 HU for positive and negative histologic group, respectively (Figure 3). Statistical difference was observed in the enhancement density between two groups (P<0.001). The ΔCT densities were studied in a ROC curve stratified to different histological findings. Figure 4 shows an area under the curve (AUC) of 0.896 (95% CI: 0.854 to 0.938; P<0.001). When setting a cut-off value of 20.5 HU, a sensitivity of 80.2% and a specificity of 89.1% were found. The AUC in the ROC curve was significantly different from 0.5, indicating that the ΔCT density has the capability to predict the positive rate in CT-guided lung biopsy specimens.

Discussion
The CT-guided biopsy is a supplementary method for detection of MTB in culture-negative TB patients (14,15). In this retrospective analysis of CT-guided lung biopsies of 356 clinically diagnosed TB patients, our data demonstrated that TB-DNA exbibits more excellent sensitivity than Z-N staining for detecting MTB from lung aspirates. Our results are comparable with studies performed on other tissue aspirates (16,17). In a recent study on lymph node aspirates from 22 patients (17), a PCR positivity of 82% was reported on the basis of the amplification of IS6110 insertion sequence compared with a positivity of 39% by Z-N staining. Therefore, our study and those of others suggest that the molecular diagnostic could make a considerable benefit in the diagnosis of pulmonary TB that are missed by conventional procedures. In addition to the superior sensitivity, another obvious advantage of molecular diagnostic is that it can distinguish between MTB and NTM, which will especially produce useful histological interpretations in the settings with high prevalence of NTM. In view of these advantages, molecular diagnostic with superiority over Z-N staining should be integrated into a clinical decision to improve the detection of tubercle bacilli from multiple aspirations.
With respect to age groups, positive histological results were less frequently seen in older patients. A recent comparative study in Tanzania has demonstrated that elderly patients are more likely to have paucibacillary TB, which is mainly due to the failure to produce good-quality of sputum in this population (18). Although the poor quality of sputum sample may be an important contributor for the low detection rate of MTB among the elderly, it could not explain our observation on the basis of CT-guided biopsy in view of intrinsic characteristics of pathological specimens. Strong evidence has revealed that immunodeficient individuals infected with MTB tend to develop paucibacillary disease, such as HIV infection and childhood TB (19,20). We therefore hypothesize that the immunosuppression status in the elderly may be associated with a low bacterial load in the lung biopsy specimens. Further experiments are urgently needed to elucidate the correlation between immunosuppression and multiplication of MTB in the lesions.
In addition, our study showed that the lung biopsies from upper lobe were more likely to yield positive histologic results than those from lower lobe. These results were consistent with previous radiographic findings that the pulmonary abnormalities are mainly located in the upper lobes, while less frequently in the lower lobe (11). In the former, the high concentration of oxygen in the upper lung areas may be associated with increased virulence of the bacilli, and enhanced multiplication of MTB (11,21). Therefore, the upper lung lobes are the favoured niche of survival for MTB, thereby resulting in the high positivity of CT-guided biopsy. From a clinical and diagnostic viewpoint, it is of great importance to be aware that the selection of lesions located in the upper lung lobes will provide more benefits for early diagnosis of pulmonary TB.
The use of contrast-enhanced CT scan has been proved to be useful to discriminate malignant nodules from benign ones (22). The enhancement on dynamic CT scans reflect the vascularity in the lesions, and the enhancement of less than 20 HU has been set as the cut-off values for the differentiation of malignant and benign nodules (22). In this study, our data demonstrated that a density-based cut-off value of 20.5 HU can be applied to help the clinicians to determine the optimal puncture site for patients with suspected TB. Considering that caseation necrosis and dystrophic calcification are the most common in almost all pulmonary TB patients (23), the fact that the TB lesions have a ΔCT density less of 20.5 HU reflects the insufficient blood supply in these areas.
There were several obvious limitations in this study. First, its retrospective nature and completion at a single center makes the universal application of the findings in the present study to different settings and populations questionable. Further randomized controlled studies are urgently needed to verify the reliability of our observation. Second, the major complication associated with CT-guided lung biopsy is an important concern that should be taken into consideration. Although a recent meta-analysis by Heerink and colleagues has revealed that major complication rate in this procedure is low (24), its risk should be carefully weighed against the benefit of potentially making a diagnosis of TB, especially for the individuals at high-risk. Despite these limitations, this study provides new insights for the integration of CT-guided lung biopsy in the clinical practice of TB diagnosis.
Conclusions
In conclusion, our data demonstrates that the molecular diagnostic has superiority over Z-N staining for detecting MTB from lung aspirates. The lung biopsies from upper lobe were more likely to yield positive histologic results than those from lower lobe. In addition, the enhancement of 20.5 HU by CT scans should be set as the cut-off values for determining the optimal puncture site that would facilitate an efficient diagnosis of pulmonary TB.
Acknowledgments
We thank all staffs from Beijing Chest Hospital for their help in carrying out this study.
Funding: This work was supported by the Beijing Talents Foundation (2017000021223ZK39) and Beijing Nova Program (Z181100006217002).
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-19-3293
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-3293). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Beijing Chest Hospital, Capital Medical University (YJS-2019008). All these patients experienced CT-guided lung biopsied after the acquisition of written informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zumla A, George A, Sharma V, et al. The WHO 2014 global tuberculosis report--further to go. Lancet Glob Health 2015;3:e10-2. [Crossref] [PubMed]
- World Health Organization (WHO). Global tuberculosis report 2018. Geneva: WHO, 2018.
- Zhao Y, Xu S, Wang L, et al. National Survey of Drug-Resistant Tuberculosis in China. New Engl J Med 2012;366:2161-70. [Crossref] [PubMed]
- Hoppe LE, Kettle R, Eisenhut M, et al. Tuberculosis--diagnosis, management, prevention, and control: summary of updated NICE guidance. BMJ 2016;352:h6747. [Crossref] [PubMed]
- Parsons LM, Somoskovi A, Gutierrez C, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev 2011;24:314-50. [Crossref] [PubMed]
- Sester M, Sotgiu G, Lange C, et al. Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J 2011;37:100-11. [Crossref] [PubMed]
- Inoue D, Gobara H, Hiraki T, et al. CT fluoroscopy-guided cutting needle biopsy of focal pure ground-glass opacity lung lesions: diagnostic yield in 83 lesions. Eur J Radiol 2012;81:354-9. [Crossref] [PubMed]
- Hiraki T, Mimura H, Gobara H, et al. CT fluoroscopy-guided biopsy of 1,000 pulmonary lesions performed with 20-gauge coaxial cutting needles: diagnostic yield and risk factors for diagnostic failure. Chest 2009;136:1612-7. [Crossref] [PubMed]
- Lee KS, Im JG. CT in adults with tuberculosis of the chest: characteristic findings and role in management. AJR Am J Roentgenol 1995;164:1361-7. [Crossref] [PubMed]
- Qin YM, Hu CY, Pang Y, et al. Saturated very-long-chain fatty acids promote cotton fiber and Arabidopsis cell elongation by activating ethylene biosynthesis. Plant Cell 2007;19:3692-704. [Crossref] [PubMed]
- Andreu J, Caceres J, Pallisa E, et al. Radiological manifestations of pulmonary tuberculosis. Eur J Radiol 2004;51:139-49. [Crossref] [PubMed]
- Hiraki T, Fujiwara H, Sakurai J, et al. Nonfatal systemic air embolism complicating percutaneous CT-guided transthoracic needle biopsy: four cases from a single institution. Chest 2007;132:684-90. [Crossref] [PubMed]
- Qin YM, Pujol FM, Hu CY, et al. Genetic and biochemical studies in yeast reveal that the cotton fibre-specific GhCER6 gene functions in fatty acid elongation. J Exp Bot 2007;58:473-81. [Crossref] [PubMed]
- Leung AN. Pulmonary tuberculosis: the essentials. Radiology 1999;210:307-22. [Crossref] [PubMed]
- Siddiqi K, Lambert ML, Walley J. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: the current evidence. Lancet Infect Dis 2003;3:288-96. [Crossref] [PubMed]
- Anand BS, Schneider FE, El-Zaatari FA, et al. Diagnosis of intestinal tuberculosis by polymerase chain reaction on endoscopic biopsy specimens. Am J Gastroenterol 1994;89:2248-9. [PubMed]
- Singh KK, Muralidhar M, Kumar A, et al. Comparison of in house polymerase chain reaction with conventional techniques for the detection of Mycobacterium tuberculosis DNA in granulomatous lymphadenopathy. J Clin Pathol 2000;53:355-61. [Crossref] [PubMed]
- Nagu T, Ray R, Munseri P, et al. Tuberculosis among the elderly in Tanzania: disease presentation and initial response to treatment. Int J Tuberc Lung Dis 2017;21:1251-7. [Crossref] [PubMed]
- Getahun H, Harrington M, O'Brien R, et al. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet 2007;369:2042-9. [Crossref] [PubMed]
- Marais BJ, Gie RP, Schaaf HS, et al. Childhood pulmonary tuberculosis: old wisdom and new challenges. Am J Respir Crit Care Med 2006;173:1078-90. [Crossref] [PubMed]
- McAdams HP, Erasmus J, Winter JA. Radiologic manifestations of pulmonary tuberculosis. Radiol Clin North Am 1995;33:655-78. [PubMed]
- Yi CA, Lee KS, Kim EA, et al. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology 2004;233:191-9. [Crossref] [PubMed]
- Lee JJ, Chong PY, Lin CB, et al. High resolution chest CT in patients with pulmonary tuberculosis: characteristic findings before and after antituberculous therapy. Eur J Radiol 2008;67:100-4. [Crossref] [PubMed]
- Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017;27:138-48. [Crossref] [PubMed]


