
Efficacy and safety of high-dose budesonide/formoterol in patients with bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplant
Introduction
Bronchiolitis obliterans syndrome (BOS) is a clinical diagnosis for the phenomenon of progressive airway obstruction after allogeneic hematopoietic stem cell transplant (HSCT) (1). BOS is characterized by progressive circumferential fibrosis and ultimate cicatrization of the small terminal airways that causes progressive dyspnea and a nonproductive cough and decreased pulmonary function characterized by an obstructive airflow pattern (2,3). Although BOS is a rare disease, probably due to challenges when making the diagnosis, its incidence is underestimated. In recent publications from Brazil (4) and Japan (5), the cumulative incidence of BOS in HSCT patients was reported to be 2.9% in 1 year, 3.7% in 3 years, and 3.43% in 5 years. The reported incidence of BOS varies among studies from 3% to 30% (6-10). In our experience, its incidence in patients who have undergone HSCT is 4.2% (11). BOS is very important to patients who have undergone HSCT because mortality and morbidity are extremely high. In a recent study of patients from 2002 to 2009, the overall survival of BOS patients was less than 55% (11). Furthermore, in another study, the 5-year survival rate of 47 HSCT recipients was 40% in those without BOS but 10% in those with it (8). Recent study has shown that the presence of BOS was associated with increased non-relapse mortality similar to patients with moderate-to-severe graft-versus-host disease (GVHD) but had no impact on overall survival (12). These studies show that the treatment and management of BOS in HSCT patients are important and that early detection and treatment result in better patient outcomes.
In a previous study, we found that budesonide/formoterol, montelukast, and N-acetylcysteine significantly improved lung function and respiratory symptoms in patients with BOS after HSCT (13). There are two types of budesonide/formoterol inhalers (160/4.5 and 320/9 µg), and the dose administered in the previous study was 160/4.5 µg. High-dose budesonide/formoterol (320/9 µg twice a day) significantly improves forced expiratory volume in 1 s (FEV1) in symptomatic asthma patients (14). Budesonide/formoterol decreases exacerbations in patients with moderate to very severe COPD (15). In 2004, a representative study of COPD showed that budesonide/formoterol 320/9.0 µg twice a day reduced the mean number of severe exacerbations by 24% and increased FEV1 by 15% vs. a placebo in patients with moderate to severe COPD (16). On the other hand, the side effects of inhaled corticosteroids (ICS) are proportional to the steroid dose (17,18). Therefore, the efficacies and adverse events of high-dose and low-dose ICS/long-acting beta agonists (LABA) need to be compared. We investigated the impact of high-dose budesonide/formoterol (320/9 µg bid) in patients with BOS after HSCT already using low-dose budesonide/formoterol (160/4.5 µg bid).
Methods
Patients
Post-HSCT patients with respiratory symptoms or a decline in pulmonary function were referred to the Pulmonology Department of the BMT Center at Seoul St. Mary’s Hospital, Seoul, South Korea. After a clinical diagnosis of BOS, one experienced pulmonologist (Rhee CK) treated all patients according to the same protocol and follow-up evaluation. There was a change in the protocol in March 2015, and the dose of budesonide/formoterol was increased from 160/4.5 to 320/9 µg twice a day in patients with BOS. After a retrospective chart review, patients who were initially treated with budesonide/formoterol 160/4.5 µg bid and increased their dose to 320/9 µg bid between March 2009 and February 2019 were enrolled.
The inclusion criteria were chronic GVHD in other organs and positive diagnostic pulmonary function tests (PFTs) using the modified NIH criteria (19) and treatment with budesonide/formoterol 160/4.5 µg bid and an increased dose to 320/9 µg bid for at least 3 months. The exclusion criteria were other pulmonary or infectious diseases, such as asthma, lung cancer, COPD, pneumonia, or tuberculosis-destroyed lungs, and a history of using other inhalers.
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional ethic committee/review board of Seoul St. Mary’s Hospital, which waived the requirement for informed consent due to the retrospective nature of the study (IRB No. KC19RESI0661).
Definition of BOS
We followed the modified NIH criteria for diagnosing BOS (19): FEV1/forced vital capacity (FVC) <0.7; FEV1 <75% of predicted with ≥10% decline over <2 years; absence of infection in the respiratory tract; one of the two supporting features of BOS (evidence of air trapping by expiratory computed tomography, or evidence of air trapping by PFTs); and residual volume (RV) >120% of predicted or RV/total lung capacity elevated outside the 90% confidence interval.
Budesonide/formoterol therapy
After diagnosing BOS, all patients enrolled were treated with 160 µg budesonide plus 4.5 µg of formoterol fumarate in a dry powder inhaler (Symbicort Turbuhaler; AstraZeneca, Molndal, Sweden) twice a day for at least 3 months and then were switched to 320 µg budesonide plus 9.0 µg formoterol fumarate twice a day. All patients were educated about the inhaler treatment. Montelukast and acetylcysteine were administered together.
PFTs
PFTs were performed before HSCT for the baseline study and after HSCT for screening and diagnosing BOS and every 3–6 months. We compared pulmonary function before and after budesonide/formoterol 160/4.5 µg therapy and before and after increasing the dose of budesonide/formoterol. We compared the PFT data collected within the prior 12 weeks and that collected after 12 weeks from the day of raising the dose.
COPD assessment test (CAT) score
The CAT score was used to measure quality of life in patients with BOS. The CAT was performed before and at least 12 weeks after the budesonide/formoterol 160/4.5 µg treatment and budesonide/formoterol 320/9.0 µg treatment. The CAT considers eight symptoms: Q1 cough, Q2 phlegm, Q3 chest tightness, Q4 breathlessness going up hills/stairs, Q5 activity limitation at home, Q6 confidence leaving home, Q7 sleep, and Q8 energy.
Defining the group responding to high-dose ICS/LABA therapy
After 3 months of high-dose budesonide plus formoterol therapy and a clinical evaluation, the patients were categorized into two groups (responder or non-responder) according to therapeutic response: above or below the minimal clinically important difference (MCID) (20). The commonly accepted MCID for pulmonary function is an FEV1 of 100 mL and a CAT score of 2 points (21,22).
Safety assessment
Safety was assessed as the incidence of pneumonia within 3 months after increasing the drug dose. Oral candidiasis, pharyngitis, pneumonia, and other adverse events were checked in the safety assessment.
Statistical analyses
Median and ranges are given for continuous variables. Categorical data are described as numbers and percentages (%). The t-test and paired t-test were performed to compare the PFT data and CAT scores. Categorical variables were compared using the chi-square or Fisher’s exact test. All statistical tests were two-sided, with P values ≤0.05 denoting significance. The statistical analyses were performed using SPSS 24 software (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
A total of 290 patients were diagnosed with BOS at Seoul St. Mary’s Hospital from March 2009 to February 2019, and 77 patients were treated with budesonide 160 µg plus formoterol 4.5 µg twice a day for more than 3 months and the dose was increased to budesonide 320 µg plus 9.0 µg twice a day. The baseline characteristics of the enrolled patients are shown in Table 1. Median age was 47.08 years (range, 22–70 years), and 67.5% of the participants were male. No current smokers were enrolled, and 28.6% were ex-smokers. Most of the patients had HSCT as treatment for acute leukemia (AML 42.9%, ALL 22.1%) or myelodysplastic syndrome (22.1%). It took a median of 654 days (40–2,973 days) to diagnose BOS. The rate of acute GVHD was 59.7%, and the rate of chronic GVHD was 100%. The eyes and oral cavity were the most frequent organs involved in chronic GVHD.
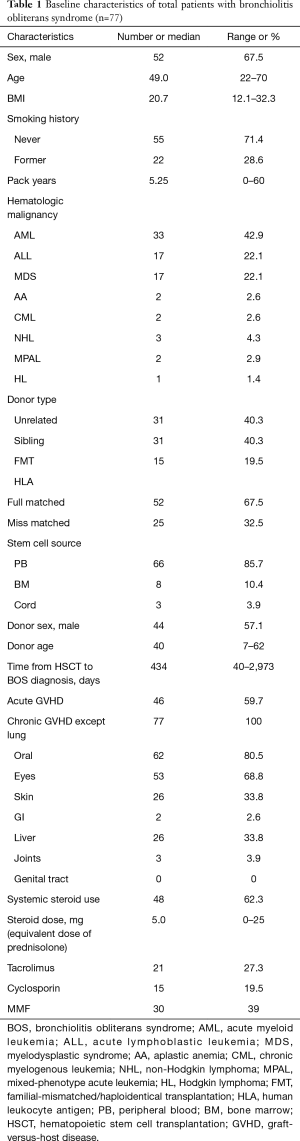
Full table
Treatment response–PFTs
After treatment with low-dose ICS/LABA (budesonide 160 µg plus formoterol 4.5 µg twice a day for 12 weeks), there were significant increases in FVC percent predicted (before 70.6% vs. after 73.8%, P=0.013) and DLCO percent predicted (before 47.9% vs. after 53.9, P=0.032). However, there were no significant differences in FEV1, FEV1 percent predicted, FEV1/FVC percent predicted and RV/TLC percent predicted. The statistical results are given in Table 2. After treatment with high-dose ICS/LABA (budesonide 320 µg plus formoterol 9.0 µg twice a day for 12 weeks), there were no significant differences in FEV1 (before treatment 1.59 L vs. after treatment 1.65 L, P=0.182) or FVC (before treatment 2.93 L vs. after treatment 2.96 L, P=0.519) compared to before starting the high dose treatment. No significant differences were observed in FEV1/FVC with the high-dose combination treatment (56.3±18.2 before and 58.2±18.4 after the high dose treatment; P=0.150). No significant differences were observed in total lung capacity (TLC) (before 5.22 L vs. after 5.17 L, P=0.646), RV (before 2.14 L vs. after 2.10 L, P=0.595), or RV/TLC (before 41.0% vs. 41.0%, P=0.901) with the high-dose combination treatment. None of these changes and improvements were significant. The statistical results are given in Table 3.

Full table
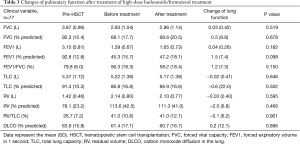
Full table
Treatment response–CAT score
Of the 77 enrolled patients, 52 completed the CAT questionnaire before and after taking low-dose ICS/LABA (budesonide/formoterol 160/4.5 µg twice a day for 12 weeks). After treatment with low-dose ICS/LABA, there was significant decrease in total CAT score (P=0.001). Total CAT score decreased from 16.2 to 13.2 after low-dose budesonide/formoterol therapy. When comparing each question, scores for question 1 (cough), question 3 (chest tightness) and question 4 (breathlessness going up hills/stairs) decreased significantly (Table 4). Sixty-seven patients completed the CAT questionnaire before and after raising their budesonide/formoterol dose to 320/9.0 µg. After raising the dose of combination therapy, there were no significant differences in the total CAT score (Table 5). The total CAT score before treatment was 16.51; it was 16.41 after treatment. When comparing each question, there was no significant decrease in the score for each question.

Full table

Full table
Change in pulmonary function in the therapeutic response group
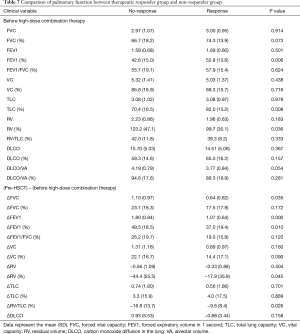
Statistical analyses were performed for therapeutic responsiveness. Of the 77 patients, 73 completed PFTs before and after the high-dose budesonide/formoterol treatment. Of these, 25 (34.2%) had an increase in FEV1 ≥100 mL. On the other hand, 68 of the 77 patients completed the CAT before and after high-dose budesonide/formoterol combination therapy. Of these, 24 (35.3%) showed a decrease ≥2 points. Among the 25 patients with increase in FEV1 ≥100 mL, 10 patients had decreased in CAT score ≥2 points, 8 patients had decreased in CAT score by 1 to −1 point and 6 patients had increased in CAT score ≥2 points. One patient did not completed CAT. Among the 24 patients with decrease in CAT score ≥2 points, 10 patients had increase in FEV1 ≥100 mL, 8 patients had increased in FEV1 by 100 to −100 mL and 6 patients had decreased in FEV1 ≥100 mL. Twenty-six (38.2%) patients were included in the therapeutic response group (Figure 1). Table 6 shows baseline characteristics of the response group and the non-response group. When compared with non-responder group, those who had response with high-dose budesonide/formoterol were significantly older (54.7 vs. 43.2, P<0.001), had a significantly longer pack-year history of smoking (10.8 vs. 2.2, P=0.034), had higher proportion of former smokers (46.2% vs. 19%, P=0.017). There were significant differences in baseline pulmonary function before HSCT when BOS did not occur. The responder group showed significantly lower FEV1 (2.76 vs. 3.28 L, P=0.010), FEV1/FVC percent predicted (76.9% vs. 81.8%, P=0.002) and significantly higher RV/TLC percent predicted (30.0 vs. 25.0, P=0.008). The pulmonary functions before high-dose budesonide/formoterol therapy, non-responder showed significantly lower FEV1 percent predicted (42.6% vs. 52.8%, P=0.006), lower VC percent predicted (70.4% vs. 82.0%, P=0.008) and significantly higher RV percent predicted (123.2% vs. 99.7%, P=0.036, Table 7). In terms of pulmonary function decline, when comparing lung function of pre-HSCT study and just before increasing the dose of budesonide/formoterol, FVC (1.10 vs. 0.64 L, P=0.035), FEV1 (1.80 vs. 1.07 L, P<0.001) and FEV1 percent predicted (49.5% vs. 37.0%, P=0.010) in the non-responder group decreased more than in the responder group. The non-responder group had more increase in RV percent predicted (44.4% vs. 17.9%, P=0.045) and RV/TLC percent predicted (16.8% vs. 9.6%, P=0.026). Table 8 shows the results.
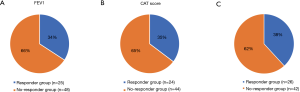
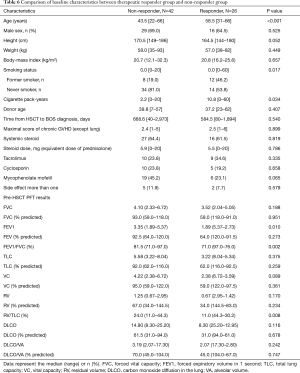
Full table
Full table

Full table
Safety assessment
In the safety assessment, 9 of the 77 patients (11.7%) developed pneumonia during the 3 months before the budesonide/formoterol 320/9 µg treatment and 8 of 77 patients (10.4%) developed pneumonia during the 3 months after the budesonide/formoterol 320/9 µg treatment, and there were no significant differences between the two periods (P=1.000). Fourteen patients (18.2%) developed bronchitis symptoms requiring antibiotics during the 3 months before the budesonide/formoterol 320/9 µg treatment and seven (9.1%) had bronchitis symptoms with antibiotics during the 3 months after the budesonide/formoterol 320/9 µg treatment and there were no significant differences between the two periods (P=0.167). Twenty-nine patients (37.7%) received antibiotic therapy for any cause during the 3 months before the budesonide/formoterol 320/9 µg treatment and twenty (26.0%) received antibiotic therapy for any cause during the 3 months after the budesonide/formoterol 320/9 µg treatment, and there were no significant differences between the two periods (P=0.078). No other complications or adverse events were reported.
Comparing pulmonary function and safety profile in control group
As a control group for the patient who raised the dose of the budesonide/formoterol from 160/4.5 to 320/9 µg, we investigated the patient group that had treatment with budesonide/formoterol 160/4.5 µg twice a day for more than 6 months. Of the 290 patients diagnosed with BOS, 77 patients who increased the dose of budesonide/formoterol from low to high were enrolled in our study. Of the remaining 213 patients, 93 patients received low-dose budesonide/formoterol inhaler treatment, 95 patients received high-dose budesonide/formoterol inhaler treatment, 16 patients received fluticasone/salmeterol inhaler treatment, and 9 received other inhaler treatments. Of 93 patients who received low-dose budesonide/formoterol treatment, 75 patients continued low-dose budesonide/formoterol treatment for more than 6 months and performed the PFTs.
We compared the differences between PFTs, CAT score, and adverse events at 3 months after 160 treatments and 6 months after these 75 patients. There were no significant differences between 3 and 6 months after low-dose budesonide/formoterol treatment in lung function, CAT score and adverse events (Tables 9-11).
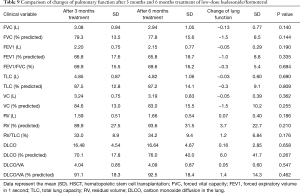
Full table
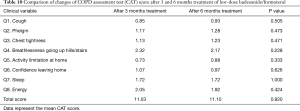
Full table

Full table
Discussion
The therapeutic effect and safety of high-dose budesonide/formoterol were investigated in patients with BOS after allogeneic HSCT. We found no significant differences in FEV1 or CAT scores between the two periods of before and after increasing the dose of budesonide/formoterol. However, some patients (34.2% in FEV1 and 35.3% in CAT score) had significant improvements that met the MICD.
The main basis of BOS therapy remains as corticosteroids (23). However, if there is no response to steroid treatment and lung function continues to deteriorate, death will ensue. In a recent study, up to a 76% 2-year survival was reported (24), but more than 60% of patients with BOS after allogeneic HSCT progress to death (8).
Bergeron et al. reported the results of ICS and bronchodilator use for BOS after allogeneic HSCT in a retrospective study (25), which sparked a randomized control trial for budesonide/formoterol performed in 32 patients that showed that budesonide/formoterol 800/24 µg twice daily significantly increased FEV1 in patients with mild/severe BOS after allogeneic HSCT but had some limitations (26). A few studies have investigated the optimal dose of budesonide/formoterol in patients with BOS after allogeneic HSCT. However, this study is the first to compare efficacies and adverse events between budesonide/formoterol 160/4.5 and 320/9.0 µg in patients with BOS after allogeneic HSCT. In a previous study, we administered a combined treatment of budesonide/formoterol 160/4.5 µg twice daily, N-acetylcysteine, and montelukast, which significantly increased FEV1 in patients with BOS after allogeneic HSCT (13). The present study has the strength of using real world data and serves as a follow-up to our previous study.
BOS is a disease in which pulmonary function continues to decrease (2,24). After the treatment of high-dose budesonide/formoterol, the CAT score and the lung function did not deteriorate in our study. It is encouraging that some patients improved over the MCID. Future research is needed to identify the predictors of the response in patients with BOS. It will be important to distinguish the phenotypes of BOS that are responsive to treatment.
The greatest concern regarding high-dose ICS was the adverse events after the increase. In this study, there were no significant differences in adverse events before and after the dose increase.
Our study had some limitations. It was not a randomized controlled study. We investigated the adverse events by retrospective chart review and there may have been under-detection of adverse events. Also, observation period for adverse events was relatively short. Finally, the responder may have had underlying reactive airway disease or early COPD before the onset of BOS. The onset of BOS may have exaggerated symptoms in some patients with potentially underlying pre-clinical COPD/asthma. The use of high-dose budesonide/formoterol may have resolved underlying pre-clinical COPD/asthma. However, there are several advantages to this study. The first was that medical care and management were standardized by a single expert using the same protocol. The second was that this is the first study to compare the effects of budesonide/formoterol 160/4 and 320/9 µg applied to patients with BOS in the real world. Finally, more than 70 patients with BOS, a very rare disease, were enrolled.
Our study failed to show superior effect of high-dose budesonide/formoterol (320/9 µg) compared with low-dose. However, high-dose budesonide/formoterol was safe and there was no lung function deterioration. Identifying subgroups of patients that have a good response to high dose budesonide/formoterol should be considered to improve the management and survival of patients with BOS after HSCT.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-19-3475
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-3475). CKR serves as an unpaid editorial board member of Journal of Thoracic Disease. CKR reports personal fees from MSD, personal fees from AstraZeneca, personal fees from GSK, personal fees from Novartis, personal fees from Takeda, personal fees from Mundipharma, personal fees from Boehringer-Ingelheim, personal fees from Teva, personal fees from Sanofi, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional ethic committee/review board of Seoul St. Mary’s Hospital, which waived the requirement for informed consent due to the retrospective nature of the study (IRB No. KC19RESI0661).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cooper JD, Billingham M, Egan T, et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transplant 1993;12:713-6. [PubMed]
- Barker AF, Bergeron A, Rom WN, et al. Obliterative bronchiolitis. N Engl J Med 2014;370:1820-8. [Crossref] [PubMed]
- Williams KM, Chien JW, Gladwin MT, et al. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA 2009;302:306-14. [Crossref] [PubMed]
- Vieira AG, Funke VA, Nunes EC, et al. Bronchiolitis obliterans in patients undergoing allogeneic hematopoietic SCT. Bone Marrow Transplant 2014;49:812-7. [Crossref] [PubMed]
- Fujii N, Nakase K, Asakura S, et al. Bronchiolitis obliterans with allogeneic hematopoietic cell transplantation: a 10-year experience of the Okayama BMT Group. Int J Hematol 2014;99:644-51. [Crossref] [PubMed]
- Gazourian L, Coronata AM, Rogers AJ, et al. Airway dilation in bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. Respir Med 2013;107:276-83. [Crossref] [PubMed]
- Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2011;17:1072-8. [Crossref] [PubMed]
- Dudek AZ, Mahaseth H, DeFor TE, et al. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant 2003;9:657-66. [Crossref] [PubMed]
- Gazourian L, Spring L, Meserve E, et al. Pulmonary Clinicopathological Correlation after Allogeneic Hematopoietic Stem Cell Transplantation: An Autopsy Series. Biol Blood Marrow Transplant 2017;23:1767-72. [Crossref] [PubMed]
- Kuzmina Z, Krenn K, Petkov V, et al. CD19(+)CD21(low) B cells and patients at risk for NIH-defined chronic graft-versus-host disease with bronchiolitis obliterans syndrome. Blood 2013;121:1886-95. [Crossref] [PubMed]
- Rhee CK, Ha JH, Yoon JH, et al. Risk Factor and Clinical Outcome of Bronchiolitis Obliterans Syndrome after Allogeneic Hematopoietic Stem Cell Transplantation. Yonsei Med J 2016;57:365-72. [Crossref] [PubMed]
- Duque-Afonso J, Ihorst G, Waterhouse M, et al. Impact of Lung Function on Bronchiolitis Obliterans Syndrome and Outcome after Allogeneic Hematopoietic Cell Transplantation with Reduced-Intensity Conditioning. Biol Blood Marrow Transplant 2018;24:2277-84. [Crossref] [PubMed]
- Kim SW, Rhee CK, Kim YJ, et al. Therapeutic effect of budesonide/formoterol, montelukast and N-acetylcysteine for bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Respir Res 2016;17:63. [Crossref] [PubMed]
- Lin CH, Hsu JY, Hsiao YH, et al. Budesonide/formoterol maintenance and reliever therapy in asthma control: acute, dose-related effects and real-life effectiveness. Respirology 2015;20:264-72. [Crossref] [PubMed]
- Calverley PM, Eriksson G, Jenkins CR, et al. Early efficacy of budesonide/formoterol in patients with moderate-to-very-severe COPD. Int J Chron Obstruct Pulmon Dis 2017;12:13-25. [Crossref] [PubMed]
- Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J 2003;21:74-81. [Crossref] [PubMed]
- Suissa S, Patenaude V, Lapi F, et al. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax 2013;68:1029-36. [Crossref] [PubMed]
- Brode SK, Campitelli MA, Kwong JC, et al. The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur Respir J 2017;50:1700037. [Crossref] [PubMed]
- Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21:389-401.e1. [Crossref] [PubMed]
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407-15. [Crossref] [PubMed]
- Donohue JF. Minimal clinically important differences in COPD lung function. Copd 2005;2:111-24. [Crossref] [PubMed]
- Kon SSC, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respiratory Medicine 2014;2:195-203. [Crossref] [PubMed]
- Uhlving HH, Buchvald F, Heilmann CJ, et al. Bronchiolitis obliterans after allo-SCT: clinical criteria and treatment options. Bone Marrow Transplant 2012;47:1020-9. [Crossref] [PubMed]
- Cheng GS, Storer B, Chien JW, et al. Lung Function Trajectory in Bronchiolitis Obliterans Syndrome after Allogeneic Hematopoietic Cell Transplant. Ann Am Thorac Soc 2016;13:1932-9. [Crossref] [PubMed]
- Bergeron A, Belle A, Chevret S, et al. Combined inhaled steroids and bronchodilatators in obstructive airway disease after allogeneic stem cell transplantation. Bone Marrow Transplant 2007;39:547-53. [Crossref] [PubMed]
- Bergeron A, Chevret S, Chagnon K, et al. Budesonide/Formoterol for bronchiolitis obliterans after hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2015;191:1242-9. [Crossref] [PubMed]


