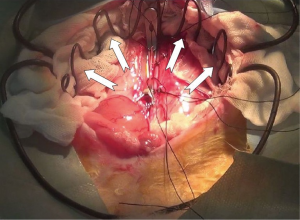
A new simplified volume-loaded heterotopic rabbit heart transplant model with improved techniques and a standard operating procedure
Introduction
Since the classic non-working (NW) heterotopic heart transplant (HTX) model in rodents was introduced by Abbott et al. (1,2) and modified by Ono et al. (3,4), this model had been widely used for researches related to immunology, graft rejection, evaluation of immunosuppressive therapies and organ preservation. Abbott et al anastomosed donor ascending aorta (AO) and pulmonary artery (PA) to the recipient abdominal aorta (AAO) and inferior vena cava (IVC) in an end-to-end fashion. Unfortunately, this technique was associated with a high mortality and graft failure rate because few animals could tolerate the occlusion of AAO. Ono and Lindsey modified the procedure and significantly improved survival rates by applying an end-to-side anastomosis between the same vessels. Since then, numerous modifications had been introduced to the basic scheme, such as “cuff technique”, cervical and femoral technique (5-7). However, the majority of models was characterized by NW and non-volume-loaded, since the left ventricle (LV) neither receives any blood from the left atrium (LA) nor pumps blood into the AO. Unloaded hemodynamics leads to myocardial atrophy and deterioration of left ventricular function, hence these models are considered not suitable for some researches (8). A few studies created a volume-loaded (VL) left heart with some complicated procedures (e.g., creation of atrial septal defect and mitral valvular regurgitation or combined heart and lung transplantation) (9-11). These methods are highly technique-dependent, or not able to apply in small animals like rats or rabbits.
Recently, the rabbit appears to be a promising model for studying pharmacology and transplant rejection for several advantages (12,13). The heart of rabbit is large enough to be divided into several segments, which is feasible to be evaluated through a variety of imaging modalities like echocardiography (ECHO), magnetic resonance imaging (MRI) or positron emission tomography-computed tomography (PET-CT) (14-16). Moreover, vascular anastomosis and manipulation can be performed rather easily without the usage of magnifier. Compared with large animal (e.g., pig or primates), rabbit models are more cost-saving, and convenient for perioperative management. We designed a new working abdominal HTX model in rabbits that is easy to perform and provides similar hemodynamic performance compared with native heart. We also developed a series of modifications on surgical techniques and perioperative managements that remarkably reduced the morbidity and increased survival rate.
Materials and methods
Animal
Thirty male allogeneic New Zealand white rabbits (Animal Laboratory of Capital Medical University, Beijing, China) weighing from 3.5 to 4 kg, served as donors and recipients for abdominal HTX. Of these, 14 (group NW) were transplanted under not-working condition (anastomoses: AAO of donor to AO of the recipient, PA of donor to IVC of the recipient), according to Ono and Lindsey (3,4). Sixteen rabbits (group VL) were performed a modified technique to create VL models. All animals received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” prepared and formulated by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publ No. 86/23, 1985). The protocol was approved by the Animal Research Committee of Beijing Anzhen Hospital. Rabbits were housed individually in stainless steel cages and maintained at 18-22 °C with a 12/12 h light/dark cycle.
Preparation and anesthesia
Animals are fasted overnight to decrease the volume of the digestive tracts so as to facilitate exposure of abdominal cavity and avert aspiration pneumonia. Preoperative fluid-restriction is not necessary. Sodium pentobarbital (Merck Inc., Germany) was administrated intravenously for anesthetic induction (30 mg/kg) and maintenance (5-10 mg/kg). Warm water bags were placed around the recipients chest (but not lower body) to avoid hypothermia. The thoracic or abdominal skin was shaved and sterilized with povidone iodine solution. All surgical procedures and instruments were complied with standard aseptic requirements. High-quality and delicate micro-instruments could facilitate vascular reconstruction and shorten operation time significantly. All donor rabbits were undergone tracheotomy, followed by endotracheal intubation and mechanical ventilation, prior to open the chest. Recipient rabbits were allowed to maintain spontaneous respiration during operation.
Donor heart harvesting
A long median sternotomy combined with a laparotomy was performed on donor rabbits with a supine position. A self-retractor was used to keep the chest open. The thymus was removed to enhance exposure of superior vena cava (SVC) and supra-aortic arch vessels. Brachiocephalic trunk (BT), aortic arch, SVC and IVC were gently isolated and passed through 4-0 silk for subsequent traction. After systemic heparinization at a dose of 125 U/kg, the BT was occluded by two bulldog clamps between its origin and bifurcation. A transverse arteriotomy was made between the two clamps, and then a heparin flushing needle connected to a cardioplegia perfusion system was inserted into the proximal part of the artery. The previous 4-0 silk around the BT was tied to fasten the needle, and the proximal clamp was released to evacuate residual air (Figure 1). At this moment, preparation for harvesting donor heart was completed. Another operation group began to perform a laparotomy on the recipient. Its infrarenal AAO and IVC were dissected for implantation.
Donor AAO was transected for exsanguination. After a few beats, heart was relatively empty. Mechanical ventilation was turned off, and SVC and IVC were occluded by two bulldog clamps. Ice-cold saline was poured into the pericardial cavity to induce cardiac arrest and enhance myocardial protection. Assistant gently massaged the heart to evacuate residual blood, and aortic arch between the BT and left carotid artery (LCA) was clamped. Cold Custodiol cardioplegia solution (Köhler Inc, Germany) at a dose of 40 mL/kg was infused into the aortic root by gravity via the heparin flushing needle. Application of a syringe to bolus inject cardioplegia is not recommended, since it will result in ventricular distension and malperfusion, or even impair LV function, secondary to aortic valve insufficiency. Subsequently, SVC and IVC was incised proximal to the site of the clamps as close as possible. The PA was also incised at the level of bifurcation to prevent cardioplegia into left heart through pulmonary circulation. At this time, operator should secure optimal cardioplegia delivery, which could be easily assessed by four indications: cardiac arrest should be quickly achieved; clear solution flow out of the incisions on SVC, IVC and PA; LV collapses, and aortic root maintain a certain tension during infusion. From now on, the cold ischemia time was recorded until the graft was placed in peritoneal cavity. During the period of cardioplegia delivery, operator began to harvest the donor heart. Aortic arch, PA, SVC and IVC were transected. Pulmonary vein and left SVC were ligated en bloc by using a 4-0 silk. Reduction of atrial volume should be avoided.
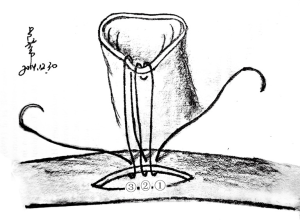
The heart was immerged in ice-cold saline for further trim. PA and IVC were ligated. A 1 cm AO and IVC stump were preserved, and spare adventitia of AO was resected to facilitate the anastomosis (In NW model, the SVC and IVC of allograft were ligated. AO and PA stump were preserved for anastomosis). Redundant stump apt to kink and torsion after vascular reconstruction, should be averted. A large and nonrestrictive shunt was created between left and right atrium by removing a part of atrial wall (Figure 2). The trabecular myocardium close to the incision were resected. We preferred a parachute technique to perform the anastomosis by using a two-arm 7-0 polypropylene suture. In most cases, this procedure can be finished within 8 minutes. The allograft did not experience warm ischemia throughout the period of harvesting. The heart was now ready for implantation.
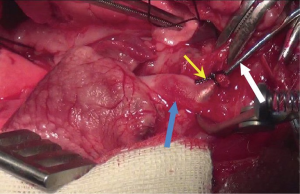
Recipients preparation
This procedure can be started at the time of donor heparinization. Prior to performing a median laparotomy, local infiltration anesthesia was achieved with 1% lidocaine hydrochloride, which significantly decreased the requirement of sodium pentobarbital. A 8-cm midline incision was made from the middle part of the abdomen to the symphysial of the pelvis. After entering the peritoneal cavity, warm, moist gauze and self-made retractor were applied to retract the colon and small intestine cranially, with care taken not to tract intestine excessively, for it may increase the risk of reflux and digestive complications. This retractor providing excellent exposure to retroperitoneum, is very easy to make (Figure S1). The retroperitoneum was open, followed by exposing AAO and IVC. The extent of dissection was limited to anastomosis site. Extensive dissection was not necessary. The site of aortotomy in AAO was proximal to the bifurcation as close as possible. However, the segment of AAO that giving off prominent lumbar arteries should not be chosen as anastomosis position. The site of venotomy in IVC was approximately 1cm cephalic to the site of aortotomy.
Graft implantation
After 125 U/kg heparin was injected via auricular vein, two of microvascular bulldog clamps were placed to occlude the IVC. The anterior wall of the IVC was grasped with two forceps. A sharp needle was used to make a pore on the IVC wall, and the pore was enlarged to a longitudinal incision which matched the size of donor SVC, with an angled micro scissor. The lumen was flushed with heparinized saline to remove any thrombus. The allograft wrapped with a cold saline gauze was placed in the right side of peritoneal cavity. From now on, the warm ischemia time was recorded until the donor heart was reperfused. The donor SVC was anastomosed to the recipient IVC in an end-to-side fashion, with a two-arm 7-0 polypropylene suture (In NW model, the donor PA was anastomosed to the recipient IVC) (Figure 2). A parachute technique with a running suture was applied to perform the anastomosis (Figure S2). Before tying down the final stitches, saline was infused into the SVC through the gap of anastomosis for de-airing of the graft. After the arterial anastomosis was completed in the same manner, the bulldog clamps placed on proximal IVC, distal IVC, distal AAO and proximal AAO were released in turns. Before restoring the proximal flow of AAO, the operator used his finger to press the arterial anastomosis, and then slowly released the clamps on the proximal AAO. This maneuver can prevent abrupt pressurization on anastomosis from tearing the stitch pore. After 10-20 s, operator removed his finger slowly to fully reperfuse the allograft and lower limb. At early stage of reperfusion, the allograft should be kept with a vertical position in order to reduce tension on the anastomosis. The maneuver can significantly decrease the incidence of hemorrhage on arterial anastomosis. In most cases, the heart will recover sinus rhythm spontaneously within 1 minute. The intestines were restored to suitable place. The abdomen was closed in two layers with interrupted suture. Finally, the animal was placed on a warm water pad to achieve normothermia, and left to recover at room temperature with unrestricted access to food and water.
Postoperative monitoring
Technically successful transplantation was defined as a functional graft for at least 5 days. The donor heart was monitored daily by palpation, whose viability was scored from 0 to 4 (17). Completely acute graft rejection was defined as palpation score 0 with the presence of anastomotic patency confirmed by ECHO. The allografts were resected when complete rejection was diagnosed. Graft survival time, daily palpation scores, the incidence of thrombosis, recipients mortality and morbidity were recorded.
ECHO
Allograft LV, aortic valve, mitral valve, and SVC parameters were evaluated daily by ECHO using a Vivid E9 system (GE Inc., US) equipped with a cardiac and vascular probe. The examiner was blinded to the experimental groups.
Positron emission tomography-computed tomography (PET-CT)
PET-CT was performed by using a Siemens Biograph mCT PET-CT system at days 3 and 6 after HTX. Freshly synthesized C2A domain of 18F-labeled Synaptotagmin I (0.4 mCi/kg) with excellent sensitivity and specificity for binding apoptotic and necrotic cell (18,19), was injected into the auricular vein. PET-CT image was captured within 60 min after injection. The standard uptake value (SUV) of the native and heterotopic heart was quantified by the imaging software of MedEy.
Statistic analysis
All quantitative data are presented as mean ± standard deviation. For statistic analysis, two-sided, unpaired Student’s t-test was used for quantitative data, and χ2 test was applied to enumeration data. Differences were considered significant when P<0.05. The analysis was performed using the statistical package for social sciences (SPSS 13.0 for windows, SPSS Inc, Chicago, Ill, US).
Result
Postoperative monitoring
The data of postoperative monitoring were shown in Table 1. There was no difference in warm ischemia time between the two groups. The cold ischemia time in group VL is longer than that in group NL, since approximate 8 min was required to create an atrial shunt. In our preliminary experiments, two recipients (2/7) died from bleeding, and one (1/7) died from pulmonary infection. No digestive complications occurred. Two recipients (2/7) developed delayed paralysis at 36 h after HTX. The AAO occlusion time in the two animals was relatively long, approximate 25 min. Moreover, the clamp sites of AAO in the two recipients were close to the origin of renal artery, but the sites in other animals were just proximal to the AAO bifurcation. After the period of learning curve, we made a series of technical modifications. In our formal experiment, no recipients suffered from paralysis, pneumonia and bleeding. Only one rabbit (1/15) in group NL died from anesthetic accident before donor heart implantation. One rabbit (1/15) in group VL presenting with anorexia, was sacrificed on postoperative day 4.

Full table
Most of allografts developed to complete cessation of ventricular contractility at day 7 after HTX. No donor hearts were able to be palpated pulse on postoperative day 10, without immunosuppressive agents (Figure S3). Recipients’ mortality and morbidity were 6.7% (1/15) and 13.3% (2/15), respectively. Without administration of anticoagulation agents, all allografts (7/7) in group NL were found thrombus in left heart when grafts were resected. This phenomenon was not found in the VL models.

ECHO
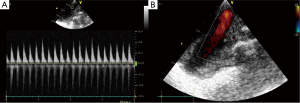
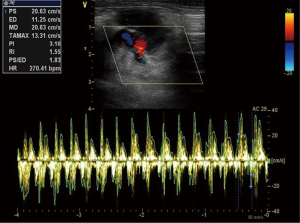
The data of ECHO were shown in Table 2. From the postoperative days 1 to 5, the aortic valve of donor hearts in group VL could open, concomitant with each LV systole (Figures S4,S5). The aortic valve of allograft in NL models maintained close during cardiac cycle. The maximal aortic velocity (MAV) of donor heart was approximately equivalent to half that of native heart in group VL. Moreover, the similar result was achieved in the parameter of late diastolic mitral inflow velocity (LDV) between donor heart and native heart in group VL (Figure 3). The ECHO showed a bidirectional flow in donor SVC of VL model, inflow during diastole and outflow during systole (Figure S6). All of donor heart in VL model had developed massive tricuspid regurgitation during right ventricular (RV) systole. A right-to-left shunt was detected through the atrial anastomosis (Figure S7).

Full table



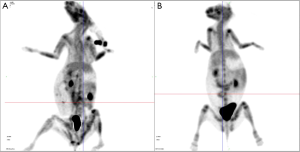
Positron emission tomography-computed tomography (PET-CT)
On the postoperative day 3, the SUV of allograft was equal to that of native heart in both groups, which indicated absence of cell death, proven by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) (not shown here). On the postoperative day 6, the donor hearts were detected an over twofold increase in SUV, because the hearts were experiencing an acute rejection (Figure 4).
Discussion
To our knowledge, this simplified VL model in rabbits was firstly introduced in our research. We also made a series of technical modifications and developed a standard operating procedure that significantly increased graft and recipient survival rate.
At present, the models for heterotopic HTX consist of cervical, femoral, and abdominal techniques. The abdominal model remains the most popular technique. Cervical and femoral technique has several advantages compared with abdominal HTX, including less vascular dissection, less blood loss, easy monitoring, short operative time and ischemia time (20). However, there is a major discrepancy in the size of the donor and recipient vessels. To solve this problem, most of studies chose a less weight animal as donor, but less weight animals with small heart will increase the difficulty to evaluate myocardial segments in some imaging modalities like MRI, PET-CT or ECHO. Some investigators closed the excessive wall of vessel to eliminate the mismatch when anastomosis. However, the vessels were more prone to twist, and thrombus in grafts is very common (21). The cuff and sleeve techniques applied in a few studies were not suitable to rabbit or larger animal model (20,22,23).
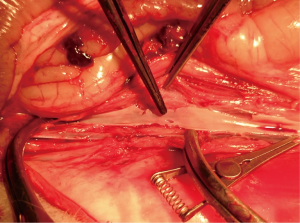
Abdominal HTX overcome these shortcomings, because large heart can be implanted into peritoneal cavity without the size mismatch of the vessels. Nevertheless, this technique is associated with digestive and nervous complications. Laden performed the classic technique modified by Ono and Lindsey on rabbits. He reported 100% incidence of paralysis unless he used young animals weighing <1 kg (24). Mitchell et al. also reported 100% incidence of paralysis in rabbit model when using this technique (25). To avoid this complication, they chose a renal artery and vein as the site of anastomosis, with resultant unilateral nephrectomy. In our preliminary experiment, we encountered two cases complicated with delayed paralysis. The autopsy demonstrated the site of anastomosis involving a prominent lumbar artery (Figure S8). It is mandatory to clamp this artery during implantation if this segment of AAO was considered as the site of anastomosis. From the orifice of left renal artery to the bifurcation, there are 5 to 7 lumbar arteries originated from AAO. The segment of infrarenal AAO close to the origin of left renal artery, usually gives off two great lumbar arteries. The diameter of the lumbar arteries close to the AAO bifurcation becomes fine. We infer postoperative paralysis is correlated with AAO clamping time and position. Accordingly, we chose a parachute technique to save the time of arterial anastomosis. Furthermore, the AAO clamping position was chosen cephalic to the bifurcation as close as possible, in order to avoid occluding prominent lumbar arteries. Compared with previous researches, these measures drastically decreased the incidence of paralysis. Moreover, we minimized disturbances to intestine by a series of protective measures like application of a self-made abdominal retractor and warm moist gauze. Our results showed a very low incidence of digestive complication.
As classic no-working model can not meet the need of some studies, a variety of modifications were performed to create an ideal VL model. The method that creating an atrial septal defect is not able to fulfill on small animal (9-11). Spencer et al. punctured the aortic valve to create regurgitation (26). This method not only produce LV distension and impair systolic function, but may fail to provide enough preload to LV. It also has the potential to reduce coronary perfusion. Wen et al. introduced a VL model by adding an LA-AAO anastomosis on the classic model (27). However, an additional anastomosis will prolong the warm ischemia time. Moreover, the fragile LA wall undergoing the pressure of systemic circulation is prone to be torn. Klima et al constructed an anastomosis between left PA and LA on a porcine donor heart to create a VL model (28). This technique is very difficult to perform on small animals.
Our VL model has several advantages compared with previous model. First, the atrial anastomosis is very easy to construct even on mouse’s hearts. This procedure will slightly prolong cold ischemia time. PET-CT imaging showed a similar SUV between NW and VL model on the postoperative day 3, which indicated slightly longer cold ischemia time did not aggravate cellular death. Second, a large and nonrestrictive shunt has the potential to provide full LV preload. Third, donor coronary sinus effluents is not able to supply adequate LV preload. The SVC-IVC connection allows blood flow back into right heart to compensate LV and RV preload (PA-IVC connection will block backflow for the existence of pulmonary valve). Fourth, a large atrial shunt can well alleviate LV distension during the period of myocardial stunning. That will facilitate the recovery of ventricular systolic function. Finally, short AAO and SVC stumps reduce the incidence of vessels twist.
Although aortic valve of allografts could open following each LV contraction under the recipient systemic pressure, the MAV and LDV were approximately equal to half that of the native heart. This indicated the hemodynamic performance of VL heart was not able to completely imitate that of native heart. We infer the impaired ventricular function was due to ischemia-reperfusion injury and acute rejection.
Conclusions
We have developed a new VL model in rabbits, which imitates a native heart hemodynamically while only requiring a minor additional procedure. Surgical technique is simple compared with currently used HTX models. We also developed a standard operating procedure that significantly improved graft and recipient survival rate. This study may be useful for investigations in transplantation in which a working model is required.
Acknowledgements
We gratefully thank Prof. Dr. Wei Fang, Director of the Department of Nuclear Medicine, Fuwai Hospital, Peking Union Medical College, who donated nuclear tracer as a gift to our study.
Disclosure: The authors declare no conflict of interest.
References
- Abbott CP, Lindsey ES, Creech O Jr, et al. A technique for heart transplantation in the rat. Arch Surg 1964;89:645-52. [PubMed]
- Abbott CP, Dewitt CW, Creech O Jr. The transplanted rat heart: histologic and electrocardiographic changes. Transplantation 1965;3:432-45. [PubMed]
- Ono K, Lindsey ES. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg 1969;57:225-9. [PubMed]
- Ono K, Lindsey ES, Creech O Jr. Transplanted rat heart: local graft irradiation. Transplantation 1969;7:176-82. [PubMed]
- Heron I. A technique for accessory cervical heart transplantation in rabbits and rats. Acta Pathol Microbiol Scand A 1971;79:366-72. [PubMed]
- Rao VK, Lisitza M. Accessory heart transplantation to groin in the rat. A new model for retransplantation experiments. Transplantation 1985;40:567-9. [PubMed]
- Gordon CR, Matthews MS, Lefebvre DR, et al. A new modified technique for heterotopic femoral heart transplantation in rats. J Surg Res 2007;139:157-63. [PubMed]
- Klein I, Samarel AM, Welikson R, et al. Heterotopic cardiac transplantation decreases the capacity for rat myocardial protein synthesis. Circ Res 1991;68:1100-7. [PubMed]
- Hoff SJ, Stewart JR, Frist WH, et al. Noninvasive detection of acute rejection in a new experimental model of heart transplantation. Ann Thorac Surg 1993;56:1074-7. [PubMed]
- Ohmi M, Yokoyama H, Nakame T, et al. Hemodynamic performance in a heterotopically transplanted dog heart: proposal of techniques for working left heart model of heterotopic (abdominal) heart transplantation. J Heart Lung Transplant 1992;11:1147-50. [PubMed]
- Campbell C, Tadros N, Heimbecker RO. Heterotopic heart-lung transplantation: a new experimental model. J Heart Transplant 1986;5:465-70. [PubMed]
- Lourenço-Filho DD, Maranhão RC, Méndez-Contreras CA, et al. An artificial nanoemulsion carrying paclitaxel decreases the transplant heart vascular disease: a study in a rabbit graft model. J Thorac Cardiovasc Surg 2011;141:1522-8. [PubMed]
- Ogawa N, Koyama I, Shibata K, et al. Hypercholesterolemia accelerates coronary artery disease after heart transplantation in a rabbit model. Transplant Proc 1994;26:2320-2. [PubMed]
- Yu M, Bozek J, Guaraldi M, et al. Cardiac imaging and safety evaluation of BMS747158, a novel PET myocardial perfusion imaging agent, in chronic myocardial compromised rabbits. J Nucl Cardiol 2010;17:631-6. [PubMed]
- Hu N, Sabey KH, Curtis HR, et al. Magnetic resonance imaging (MRI) assessment of ventricular remodeling after myocardial infarction in rabbits. Comp Med 2012;62:116-23. [PubMed]
- Casamian-Sorrosal D, Saunders R, Browne W, et al. Left ventricular radial colour and longitudinal pulsed-wave tissue Doppler echocardiography in 39 healthy domestic pet rabbits. Res Vet Sci 2014;97:376-81. [PubMed]
- Abbott CP, Dewitt CW, Creech O Jr. The transplanted rat heart: histologic and electrocardiographic changes. Transplantation 1965;3:432-45. [PubMed]
- Alam IS, Neves AA, Witney TH, et al. Comparison of the C2A domain of synaptotagmin-I and annexin-V as probes for detecting cell death. Bioconjug Chem 2010;21:884-91. [PubMed]
- Zhao M, Zhu X, Ji S, et al. 99mTc-labeled C2A domain of synaptotagmin I as a target-specific molecular probe for noninvasive imaging of acute myocardial infarction. J Nucl Med 2006;47:1367-74. [PubMed]
- Fensterer TF, Miller CJ, Perez-Abadia G, et al. Novel cuff design to facilitate anastomosis of small vessels during cervical heterotopic heart transplantation in rats. Comp Med 2014;64:293-9. [PubMed]
- Ma Y, Wang G. Comparison of 2 heterotopic heart transplant techniques in rats: cervical and abdominal heart. Exp Clin Transplant 2011;9:128-33. [PubMed]
- Li C, Luo L, Lu J, et al. A modified splint tubing technique for heterotopic heart transplantation in mouse. Transpl Immunol 2011;25:82-7. [PubMed]
- Fang J, He L, Wang SQ, et al. A simplified two-stitch sleeve technique for arterial anastomosis of cervical heterotopic cardiac transplantation in mice. Am J Transl Res 2013;5:521-9. [PubMed]
- Laden AM. Experimental atherosclerosis in rat and rabbit cardiac allografts. Arch Pathol 1972;93:240-5. [PubMed]
- Mitchell SV, Mottram PL, Purcell LJ, et al. A rabbit model for heterotopic cardiac transplantation. Transplantation 1990;49:835-7. [PubMed]
- Spencer AU, Hart JP, Cabreriza SE, et al. Aortic regurgitation in the heterotopic rat heart transplant: effect on ventricular remodeling and diastolic functio. J Heart Lung Transplant 2003;22:937-45. [PubMed]
- Wen P, Wang X, Wang J, et al. A simple technique for a new working heterotopic heart transplantation model in rats. Transplant Proc 2013;45:2522-6. [PubMed]
- Klima U, Guerrero JL, Levine RA, et al. A new, biventricular working heterotopic heart transplant model: anatomic and physiologic considerations. Transplantation 1997;64:215-22. [PubMed]
- Lu W, Zheng J, Pan XD, et al. The anterior dopplor flow when the donor heart systoles, and the backflow when donor heart diastoles. Asvide 2015;2:020. Available online: http://www.asvide.com/articles/475
- Lu W, Zheng J, Pan XD, et al. A massive right-to-left shunt in volume-loaded donor heart. Asvide 22015;2:021. Available online: http://www.asvide.com/articles/476