Fatal toxic effects related to EGFR tyrosine kinase inhibitors based on 53 cohorts with 9,569 participants
Introduction
Lung cancer is the most frequent malignancy and the leading cause of cancer death (1). Over the decade, the management of treatment options for non-small cell lung cancer (NSCLC) has continually evolved. Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), comprising erlotinib, gefitinib, afatinib, dacomitinib and osimertinib, were primary regimens for NSCLC with EGFR mutation, which has significantly prolonged progression-free survival compared with platinum-based chemotherapy (2,3) and generally coupled with tolerable adverse events like rash and diarrhea (4,5). Despite these benefits, however, fatal toxic effects might occasionally occur in individuals treated with EGFR-TKIs.
The majority of prior studies on severe toxic effects involved pulmonary toxicities, especially interstitial lung disease (ILD), and the incidence was approximately 0.20–1.00% (6-8). The minority of prior studies provided various severe causes, including hepatotoxicity, dyspnea, sepsis, and unknown cause (9,10). Additionally, a meta-analysis on both lung cancer and other cancers exhibited that the overall incidence of fatal toxic effects was 1.9% (11). However, the aforementioned studies hardly provided the precise incidence, the comprehensive spectrum and the susceptible factors of fatal toxic effects related to EGFR-TKIs in patients with NSCLC. Consequently, it is imperative to systematically estimate the incidence and provide a detailed spectrum as well as susceptible factors of fatal toxic effects related to EGFR-TKIs through extensive databases.
Herein, we performed a meta-analysis of patients with NSCLC treated with EGFR-TKIs, comprising erlotinib, gefitinib, afatinib, dacomitinib and osimertinib, to determine the accurate incidence, comprehensive spectrum and susceptible factors of fatal toxic effects related to EGFR-TKIs.
Methods
Literature search
PubMed and Embase were thoroughly searched for clinical trials based on the following terms and corresponding Medical Subject Heading ones: “erlotinib”, “gefitinib”, “afatinib”, “dacomitinib”, “osimertinib” and “NSCLC” before October 25, 2018.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (I) study on NSCLC treated with EGFR-TKIs, including erlotinib, gefitinib, afatinib, dacomitinib and osimertinib; (II) availability of fatal toxicity results; (III) articles published in English. On the contrary, the exclusion criteria were as follows: (I) fatal toxic effects related to EGFR-TKIs unavailable; (II) trial phase undefined; (III) full texts unavailable; (IV) other agents combined; (V) retrospective articles.
Data extraction
The type of EGFR-TKIs, generation of drugs, trial phase of studies, treatment-line, EGFR status, study regions, average age, the number and type of fatal causes, and the total number of patients were extracted from eligible articles. Defining 63 as a cut-off age since it was the median age of eligible studies. The treatment-line was assigned into three groups as follows: (I) first-line treatment group: without any systematic treatment; (II) prior chemotherapy group: treated with EGFR-TKI following chemotherapy; (III) EGFR-TKI retreatment group: treated with distinct types of EGFR-TKI following EGFR-TKI therapy. Fatal toxic effects related to EGFR-TKIs were defined as that death was merely attributed to drugs rather than disease progression or ambiguous reasons. To accommodate the different terminology used in various studies, pneumonitis and interstitial pneumonitis were categorized into ILD, aspirational pneumonia and lung infection into pneumonia and respiratory decompensation into respiratory failure.
Screening of eligible articles and extracting of data was conducted individually by two reviewers, conforming to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (12). Any discrepancy was resolved by a third researcher.
Statistical methods
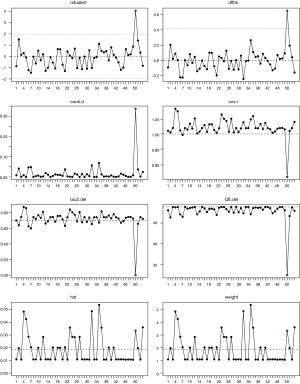
The study was focused on the evaluation of rare events (the incidence of fatal toxic effects was far below 20%). Thus, the raw data was conformed to a normal distribution by logit transformation for improving the validity of the analysis (13). Mixed-effects logistic regression was used for calculating pooled incidence and corresponding 95% confidence intervals (CI) of fatal toxic effects related to EGFR-TKIs. Subgroup analyses were performed based on EGFR-TKI agents, generation of the drug, trial phase of studies, treatment-line, EGFR status, study regions and average age (P<0.05 indicated statistical significance). Heterogeneity was assessed by Higgins inconsistency index (I2) test and values higher than 50% implied substantial heterogeneity (14). The univariate meta-regression analysis was carried out to estimate the correlation between various covariates and the incidence of fatal toxic effects related to EGFR-TKIs. The multivariate meta-regression analysis was performed to distinguish susceptible factors from diverse variables, comprising a value of P<0.05 that occurred in univariate meta-regression analysis and important clinical factors. Publication bias was evaluated by funnel plot and Egger’s or Begg’s tests (15). Potential outliers were identified by the value of externally studentized residuals, which greater than 2 indicated outliers (16), and influential studies would be marked with red in the influence plot.
Pooled analyses were conducted by the “metafor” and “meta” packages in R version 3.4.4 (R foundation).
Results
Eligible studies and characteristics
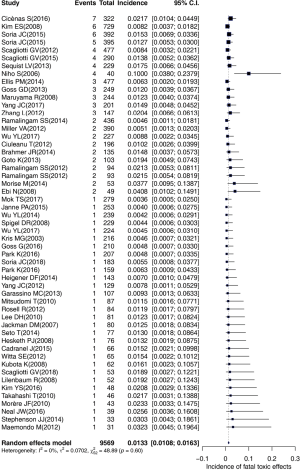
A total of 1,904 records were screened and evaluated for eligibility, and 50 studies, involving erlotinib (17-39), afatinib (40-45) and other EGFR-TKIs (3,46-65). Finally, 53 cohorts with 9,569 participants were identified (Figure 1). Totally, 105 cases of fatal toxic effects related to EGFR-TKIs occurred in 53 cohorts. All studies were prospective clinical trials.
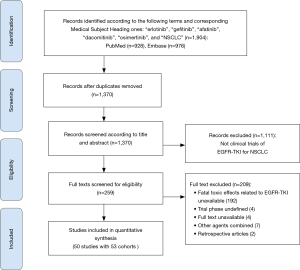
A study with phase II/III was categorized into phase III study (44), which had two deaths related to afatinib. Besides, 18 cases of fatal toxic effects related to EGFR-TKIs occurred in three studies incorporating six cohorts (42,49,62). Only one study was phase I (46), which possessed one death related to osimertinib. Erlotinib was dominantly used, and first-generation EGFR-TKI composed of erlotinib and gefitinib was frequently used in 53 cohorts. Detailed characteristics of 53 eligible cohorts were presented in Table 1.
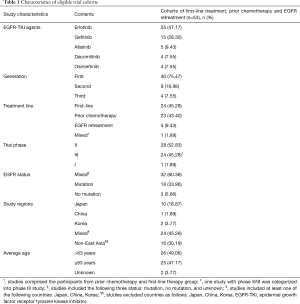
Full table
Incidence of fatal toxic effects related to EGFR-TKIs
The overall incidence of fatal toxic effects related to EGFR-TKIs was 1.33% (95% CI: 1.08–1.63%). Heterogeneity was not observed in our study (I2=0%, P=0.60). Subgroup analyses were performed based on EGFR-TKI agents, generation of the drug, trial phase of studies, treatment-line, EGFR status, study regions and average age. The results were presented in Table 2. Notably, the incidence was apparently higher in the Japanese group (compared with the non-East Asian group) (2.72% vs. 1.30%, P=0.015), in first-line treatment group (compared with EGFR-TKI retreatment group) (1.54% vs. 0.69%, P=0.028), and in the trial phase II (compared with trial phase III) (1.82% vs. 1.11%, P=0.009). No significant distinction was observed for the incidence among Asian groups without Japanese, the type of EGFR-TKIs, generation of drugs, EGFR status, and average age.
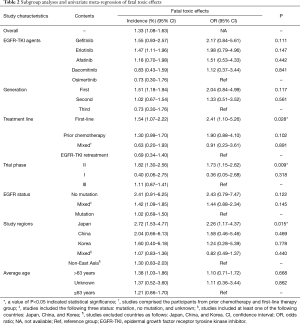
Full table
The results of univariate meta-regression analysis showed that the odds were evidently higher in the Japanese group than non-East Asian group [odds ratio (OR): 2.26, 95% CI: 1.17–4.37, P=0.015], higher in first-line treatment group than EGFR-TKI retreatment group (OR: 2.41, 95% CI: 1.10–5.26, P=0.028), and higher in the trial phase II than trial phase III (OR: 1.73, 95% CI: 1.15–2.62, P=0.009). No significant distinction for the odds among the type of EGFR-TKIs, generation of drugs, EGFR status and average age. The detailed results were showed in Table 2.
The three factors (study regions, the trial phase, and the treatment-line) were included in multivariate meta-regression analysis for identifying susceptible factors. The detailed results were presented in Table 3. Impressively, the Japanese group had a markedly higher incidence than non-East Asian group after controlling the treatment-line and trial phase (OR: 2.16, 95% CI: 1.12–4.16, P=0.022). However, no significant discrepancy existed in the trial phase II and phase III after adjusting to the treatment-line and study regions. Additionally, a similar trend was found in different treatment-line group following controlling for study regions and the trial phase.

Full table
A potential outlier was noted by the forest plot of the overall incidence presented in Figure 2. The value of externally studentized residuals of the study (8) was larger than 2 (z=4.05). Thus, it was regarded as a potential outlier. To determine whether the study might impact the overall incidence, we performed a plot of influence and the influential study would be marked with red (Figure 3). Thus, the study (52) was regarded as influential, and the re-estimated incidence was 1.25% when the study (52) was removed from the 53 cohorts.
The spectrum of fatal toxic effects related to EGFR-TKIs
To provide a comprehensive spectrum of fatal toxic effects related to EGFR-TKIs, we thoroughly evaluated the types among 105 fatal cases. A total of 29 fatal causes were documented, among which ILD was highly predominant. Subsequent fatal causes were as follows: pneumonia, respiratory failure, diarrhea, hemoptysis, pulmonary infiltrates, hepatic and renal failure, heart failure and unknown cause. Moreover, we noted the respiratory system was most frequently involved, followed by the digestive system. A detailed spectrum of fatal toxic effects related to EGFR-TKIs was presented in Table 4. Notably, ILD was the most frequent fatal cause regardless of various regimens.
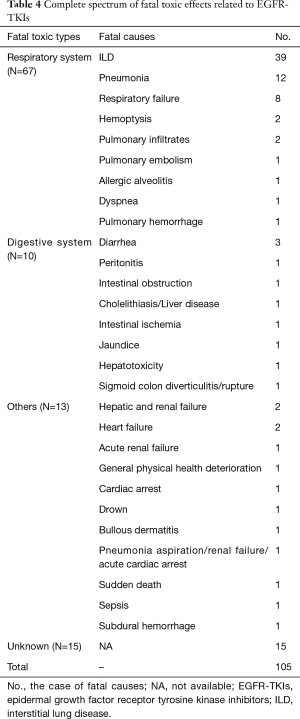
Full table
Publication bias
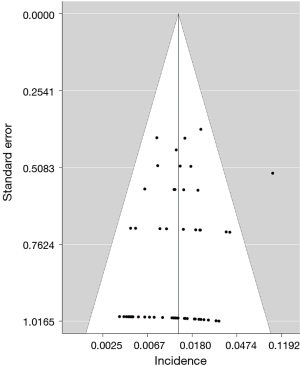
The funnel plot of the incidence of fatal toxic effects related to EGFR-TKIs was asymmetric (Figure 4). However, no evidence of publication bias was provided for the incidence of fatal toxic effects according to Egger’s test (P=0.144).
Discussion
To the best of our knowledge, this study provided the most comprehensive analysis of fatal toxic effects related to EGFR-TKIs through widespread databases. The overall incidence was 1.33%, which was significantly higher in the Japanese group (compared with the non-East Asian group), in the first-line treatment group (compared with the EGFR-TKI retreatment group), and in the trial phase II (compared with trial phase III). The susceptible factor of fatal toxic effects related to EGFR-TKIs was the Japanese group. ILD was predominant fatal cause regarding different agents.
Despite the number of fatal toxic effects related to EGFR-TKIs was notable (n=105), the incidence was rare for patients with NSCLC (1.33%). The result was different from the prior study including 15 trials whose incidence was 1.7% (66), which might derive from diverse amounts of eligible studies. Moreover, dominant fatal cause was ILD, which was analogous to that of prior study (10). However, this study firstly provided the most detailed spectrum and susceptible factors of fatal toxic effects related to EGFR-TKIs based on widespread databases.
Although the precise interpretation of a higher incidence of fatal toxic effects for the Japanese group remained unclear, the result might be attributed to environmental factors and genetic polymorphisms (67). Consequently, it was crucial to further explore the underlying mechanism. Compared with the first-line treatment group, we found that individuals repeatedly used EGFR-TKIs might less likely to suffer from a drug-related death. Intriguingly, participants enrolled in the trial phase II possessed a higher incidence than trial phase III. The possible interpretation was that the design of subsequent trials was optimized according to the experience of early trials to facilitate the contraction of incidence. Additionally, we scrutinized the 53 cohorts and found that the majority of studies on the Japanese group were trial phase II (8/10). Thus, it should be cautious to interpret the result. Furthermore, we also noted the respiratory system was most frequently involved. Therefore, pulmonary adverse events occurring in patients with EGFR-TKIs treatment were needed to be handled as soon as possible. Further researches should be taken to minimize the fatal toxic effects related to EGFR-TKIs.
Regardless of the outlier that was observed in this study, we remained to enroll the study (52) which included potential predisposing factors of fatal toxic effects related to EGFR-TKIs (Japanese group, first-line treatment group and the trial phase II). The incidence of fatal toxic effects on a single study was 10%, which significantly higher than the overall incidence (1.33%). Therefore, if we deleted the study (52), the credibility of the results might be undermined.
Some limitations we encountered were as follows: firstly, these events we estimated were rare and incidence was far below 20%, thus we thoroughly screened and evaluated eligible studies via widespread database; secondly, the causality between drugs and fatal toxic effects was not clearly stated in several studies, hence we had to select the studies that definitely stated fatal causes were attributed to drugs rather than disease progression or ambiguous reasons; finally, as unknown cause occurred in several studies, which hindered detailed analysis of fatal toxic effects.
In conclusion, the overall incidence of fatal toxic effects related to EGFR-TKIs was 1.33%, and the major fatal cause was ILD followed by pneumonia and respiratory failure. The pulmonary system was the most frequently involved. The susceptible factor of fatal toxic effects related to EGFR-TKIs was the Japanese group. The study provided a capability for clinicians to predict and detect high-risk populations of fatal toxic effects.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-4000A). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Natale RB, Thongprasert S, Greco FA, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2011;29:1059-66. [Crossref] [PubMed]
- Suzumura T, Kimura T, Kudoh S, et al. Reduced CYP2D6 function is associated with gefitinib-induced rash in patients with non-small cell lung cancer. BMC Cancer 2012;12:568. [Crossref] [PubMed]
- Akamatsu H, Inoue A, Mitsudomi T, et al. Interstitial lung disease associated with gefitinib in Japanese patients with EGFR-mutated non-small-cell lung cancer: combined analysis of two Phase III trials (NEJ 002 and WJTOG 3405). Jpn J Clin Oncol 2013;43:664-8. [Crossref] [PubMed]
- Qi WX, Sun YJ, Shen Z, et al. Risk of interstitial lung disease associated with EGFR-TKIs in advanced non-small-cell lung cancer: a meta-analysis of 24 phase III clinical trials. J Chemother 2015;27:40-51. [Crossref] [PubMed]
- Shi L, Tang J, Tong L, et al. Risk of interstitial lung disease with gefitinib and erlotinib in advanced non-small cell lung cancer: a systematic review and meta-analysis of clinical trials. Lung Cancer 2014;83:231-9. [Crossref] [PubMed]
- Wang Y, Wang M, Wang Q, et al. Incidence and risk of infections associated with EGFR-TKIs in advanced non-small-cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Oncotarget 2017;8:29406-15. [Crossref] [PubMed]
- Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer 2015;88:74-9. [Crossref] [PubMed]
- Qi WX, Tang LN, He AN, et al. Incidence and risk of treatment-related mortality in cancer patients treated with EGFR-TKIs: a meta-analysis of 22 phase III randomized controlled trials. Respir Med 2013;107:1280-3. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65-94. [Crossref] [PubMed]
- Barendregt JJ, Doi SA, Lee YY, et al. Meta-analysis of prevalence. J Epidemiol Community Health 2013;67:974-8. [Crossref] [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods 2010;1:112-25. [Crossref] [PubMed]
- Brahmer JR, Lee JW, Traynor AM, et al. Dosing to rash: a phase II trial of the first-line erlotinib for patients with advanced non-small-cell lung cancer an Eastern Cooperative Oncology Group Study (E3503). Eur J Cancer 2014;50:302-8. [Crossref] [PubMed]
- Kubota K, Nishiwaki Y, Tamura T, et al. Efficacy and safety of erlotinib monotherapy for Japanese patients with advanced non-small cell lung cancer: a phase II study. J Thorac Oncol 2008;3:1439-45. [Crossref] [PubMed]
- Ciuleanu T, Stelmakh L, Cicenas S, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol 2012;13:300-8. [Crossref] [PubMed]
- Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236-44. [Crossref] [PubMed]
- Cadranel J, Gervais R, Merle P, et al. Erlotinib versus carboplatin and paclitaxel in advanced lepidic adenocarcinoma: IFCT-0504. Eur Respir J 2015;46:1440-50. [Crossref] [PubMed]
- Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013;14:981-8. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Neal JW, Dahlberg SE, Wakelee HA, et al. Erlotinib, cabozantinib, or erlotinib plus cabozantinib as second-line or third-line treatment of patients with EGFR wild-type advanced non-small-cell lung cancer (ECOG-ACRIN 1512): a randomised, controlled, open-label, multicentre, phase 2 trial. Lancet Oncol 2016;17:1661-71. [Crossref] [PubMed]
- Spigel DR, Lin M, O'Neill V, et al. Final survival and safety results from a multicenter, open-label, phase 3b trial of erlotinib in patients with advanced nonsmall cell lung cancer. Cancer 2008;112:2749-55. [Crossref] [PubMed]
- Park K, Yu CJ, Kim SW, et al. First-Line Erlotinib Therapy Until and Beyond Response Evaluation Criteria in Solid Tumors Progression in Asian Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer: The ASPIRATION Study. JAMA Oncol 2016;2:305-12. [Crossref] [PubMed]
- Cicènas S, Geater SL, Petrov P, et al. Maintenance erlotinib versus erlotinib at disease progression in patients with advanced non-small-cell lung cancer who have not progressed following platinum-based chemotherapy (IUNO study). Lung Cancer 2016;102:30-7. [Crossref] [PubMed]
- Heigener DF, Deppermann KM, Pawel JV, et al. Open, randomized, multi-center phase II study comparing efficacy and tolerability of Erlotinib vs. Carboplatin/Vinorelbin in elderly patients (>70 years of age) with untreated non-small cell lung cancer. Lung Cancer 2014;84:62-6. [Crossref] [PubMed]
- Jackman DM, Yeap BY, Lindeman NI, et al. Phase II clinical trial of chemotherapy-naive patients > or = 70 years of age treated with erlotinib for advanced non-small-cell lung cancer. J Clin Oncol 2007;25:760-6. [Crossref] [PubMed]
- Morise M, Taniguchi H, Saka H, et al. Phase II study of erlotinib for previously treated patients with EGFR wild-type non-small-cell lung cancer, following EGFR mutation status reevaluation with the Scorpion Amplified Refractory Mutation System. Mol Clin Oncol 2014;2:991-6. [Crossref] [PubMed]
- Takahashi T, Yamamoto N, Nukiwa T, et al. Phase II study of erlotinib in Japanese patients with advanced non-small cell lung cancer. Anticancer Res 2010;30:557-63. [PubMed]
- Goto K, Nishio M, Yamamoto N, et al. A prospective, phase II, open-label study (JO22903) of first-line erlotinib in Japanese patients with epidermal growth factor receptor (EGFR) mutation-positive advanced non-small-cell lung cancer (NSCLC). Lung Cancer 2013;82:109-14. [Crossref] [PubMed]
- Stephenson JJ, Nemunaitis J, Joy AA, et al. Randomized phase 2 study of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus erlotinib in patients with non-small cell lung cancer. Lung Cancer 2014;83:219-23. [Crossref] [PubMed]
- Lilenbaum R, Axelrod R, Thomas S, et al. Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol 2008;26:863-9. [Crossref] [PubMed]
- Witta SE, Jotte RM, Konduri K, et al. Randomized phase II trial of erlotinib with and without entinostat in patients with advanced non-small-cell lung cancer who progressed on prior chemotherapy. J Clin Oncol 2012;30:2248-55. [Crossref] [PubMed]
- Scagliotti GV, Bondarenko I, Blackhall F, et al. Randomized, phase III trial of figitumumab in combination with erlotinib versus erlotinib alone in patients with nonadenocarcinoma nonsmall-cell lung cancer. Ann Oncol 2015;26:497-504. [Crossref] [PubMed]
- Hesketh PJ, Chansky K, Wozniak AJ, et al. Southwest Oncology Group phase II trial (S0341) of erlotinib (OSI-774) in patients with advanced non-small cell lung cancer and a performance status of 2. J Thorac Oncol 2008;3:1026-31. [Crossref] [PubMed]
- Scagliotti GV, Krzakowski M, Szczesna A, et al. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a phase III trial. J Clin Oncol 2012;30:2070-8. [Crossref] [PubMed]
- Scagliotti GV, Shuster D, Orlov S, et al. Tivantinib in Combination with Erlotinib versus Erlotinib Alone for EGFR-Mutant NSCLC: An Exploratory Analysis of the Phase 3 MARQUEE Study. J Thorac Oncol 2018;13:849-54. [Crossref] [PubMed]
- Yang JC, Shih JY, Su WC, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol 2012;13:539-48. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Soria JC, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015;16:897-907. [Crossref] [PubMed]
- Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. [Crossref] [PubMed]
- Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012;13:528-38. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Ellis PM, Shepherd FA, Millward M, et al. Dacomitinib compared with placebo in pretreated patients with advanced or metastatic non-small-cell lung cancer (NCIC CTG BR.26): a double-blind, randomised, phase 3 trial. Lancet Oncol 2014;15:1379-88. [Crossref] [PubMed]
- Ramalingam SS, Janne PA, Mok T, et al. Dacomitinib versus erlotinib in patients with advanced-stage, previously treated non-small-cell lung cancer (ARCHER 1009): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:1369-78. [Crossref] [PubMed]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454-66. [Crossref] [PubMed]
- Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 2003;290:2149-58. [Crossref] [PubMed]
- Maemondo M, Minegishi Y, Inoue A, et al. First-line gefitinib in patients aged 75 or older with advanced non-small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 study. J Thorac Oncol 2012;7:1417-22. Erratum in: J Thorac Oncol 2012;7:e33. Gemmah, Akihiko [corrected to Gemma, Akihiko]. [Crossref] [PubMed]
- Niho S, Kubota K, Goto K, et al. First-line single agent treatment with gefitinib in patients with advanced non-small-cell lung cancer: a phase II study. J Clin Oncol 2006;24:64-9. [Crossref] [PubMed]
- Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008;372:1809-18. [Crossref] [PubMed]
- Zhang L, Ma S, Song X, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol 2012;13:466-75. [Crossref] [PubMed]
- Goss GD, O'Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013;31:3320-6. [Crossref] [PubMed]
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643-52. [Crossref] [PubMed]
- Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in Pretreated T790M-Positive Advanced Non-Small-Cell Lung Cancer: AURA Study Phase II Extension Component. J Clin Oncol 2017;35:1288-96. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Ebi N, Semba H, Tokunaga SJ, et al. A phase II trial of gefitinib monotherapy in chemotherapy-naive patients of 75 years or older with advanced non-small cell lung cancer. J Thorac Oncol 2008;3:1166-71. [Crossref] [PubMed]
- Maruyama R, Nishiwaki Y, Tamura T, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 2008;26:4244-52. [Crossref] [PubMed]
- Ramalingam SS, Blackhall F, Krzakowski M, et al. Randomized phase II study of dacomitinib (PF-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 2012;30:3337-44. [Crossref] [PubMed]
- Kim YS, Cho EK, Woo HS, et al. Randomized Phase II Study of Pemetrexed Versus Gefitinib in Previously Treated Patients with Advanced Non-small Cell Lung Cancer. Cancer Res Treat 2016;48:80-7. [Crossref] [PubMed]
- Morère JF, Brechot JM, Westeel V, et al. Randomized phase II trial of gefitinib or gemcitabine or docetaxel chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2 or 3 (IFCT-0301 study). Lung Cancer 2010;70:301-7. [Crossref] [PubMed]
- Lee DH, Park K, Kim JH, et al. Randomized Phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res 2010;16:1307-14. [Crossref] [PubMed]
- Ding PN, Lord SJ, Gebski V, et al. Risk of Treatment-Related Toxicities from EGFR Tyrosine Kinase Inhibitors: A Meta-analysis of Clinical Trials of Gefitinib, Erlotinib, and Afatinib in Advanced EGFR-Mutated Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:633-43. [Crossref] [PubMed]
- Nyberg F, Barratt BJ, Mushiroda T, et al. Interstitial lung disease in gefitinib-treated Japanese patients with non-small-cell lung cancer: genome-wide analysis of genetic data. Pharmacogenomics 2011;12:965-75. [Crossref] [PubMed]