Utility of the sliding lung sign for the prediction of preoperative intrathoracic adhesions
Introduction
Unexpected intrathoracic adhesions are relatively uncommon complications of thoracic surgery. Severe adhesion can reduce the working space for the surgeon, leading to increased operative time and greater risks of complications and organ injury (1-3). Video-assisted thoracoscopic surgery (VATS) is an approach that may offer a solution to these issues during thoracotomy. In addition to alleviating the stress on the surgeon and other staff, it may improve operative times and patient safety (4-6). These lines of evidence suggest that preoperative prediction of intrathoracic adhesions is important to plan the surgical approach and to implement appropriate safety management.
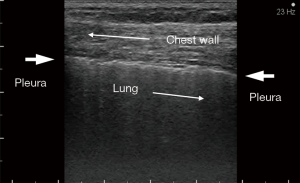
In standard practice, intrathoracic adhesions are empirically predicted from X-ray data and past surgical history, while chest-wall invasion of lung cancer can be effectively evaluated using ultrasonography and cine magnetic resonance imaging (MRI) (7-10), based on changes in lung respiration. The presence of the lung respiratory changes can be evaluated as the “sliding lung sign” by chest ultrasonography (Figure 1) (11); it has been reported to be useful not only for evaluating pleural effusions and chest wall invasion (7,9,10,12,13), but also for evaluating the development of pneumothorax, diagnosing interstitial lung disease, acute respiratory failure, guiding lung biopsy, for endotracheal tube placement, and for planning pleurodesis (11,14-18). We believe that the sliding lung sign might also predict intrathoracic adhesion. Several studies have reported the utility of ultrasonography for determining intrathoracic adhesions (18-22). However, they were mainly retrospective studies, with limited representation of adhesion characteristics and comparisons with factors such as restrictive lung disorder, blunting of the costophrenic (CP) angle, computed tomography (CT) findings, past medical history, as well as small sample sizes. There was also little information regarding perioperative variables.
The present prospective, observational study investigated the utility of the sliding lung sign to preoperatively predict intrathoracic severe adhesion, and compared the outcomes with those of other modalities. We also measured the risks of postoperative complications and the factors predictive of severe intrathoracic adhesions.
We present the following article in accordance with the STROBE reporting checklist (available at
Methods
Study design and patients
This was a single center, prospective, observational study (UMIN 000039054). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study and all its protocols were approved by the Ethics Committee of the Joetsu General Hospital (J-70). All patients provided written informed consent. We included all patients aged 20–89 years who were admitted to Joetsu General Hospital (Joetsu, Niigata, Japan) for elective thoracic surgery between April 2013 and December 2015. Exclusion criteria were as follows: pneumothorax, hydrothorax, hemothorax, pyothorax, chylothorax, age of ≤19 or ≥90 years, median sternotomy, psychiatric disorders that inhibited participation, and inappropriate participation. Participants with histories of thoracic surgery were included.
Chest ultrasound procedure
Chest ultrasound sonography was performed at our outpatient clinic prior to surgery. The presence of the sliding lung sign was determined using B-mode imaging on each intercostal space of the chest (Figure 1). The movement of the visceral pleural slide were measured uniformly from the front to the lateral and the back side during exaggerated respiration. The evaluations in the upper side were difficult due to the narrower intercostal space and the scapula. Therefore, measurements in the upper side were performed within the observable range. A linear-type ultrasound probe (7.5 MHz) was used with a Prosound α7 scanner (Hitachi-Aloka medical, Ltd. Tokyo, Japan). Chest ultrasound sonography was mainly performed by the chief surgeon, who evaluated all data.
Diagnosis of severe intrathoracic adhesions
Severe intrathoracic adhesion was defined as the need for adhesiolysis requiring more than 30 minutes. Operative data were also recorded in detail in video and anesthesia chart formats.
Surgical procedure
General anesthesia was maintained using single-lung ventilation with a double-lumen endotracheal tube. Patients were placed in the lateral decubitus position. The surgical approach first involved complete VATS. Patients requiring partial lung resection, including bullectomy, underwent 3-port VATS with two 5-mm ports and one 10-mm port. For lobectomy and segmentectomy, an additional 5-mm port was used. Patients with locally advanced carcinoma underwent VATS using mini-thoracotomy or open thoracotomy. Three surgeons performed the surgeries; complicated surgeries were performed by the chief surgeon. A 20-Fr chest tube was inserted at the end of the procedure.
Variables and assessments
The following patient, ultrasound, and surgical characteristics and follow-up parameters were recorded: age, gender, smoking history, history of exposure to asbestos, interstitial pneumonia, past medical history (defined as “positive” in the case of previous pneumonia, pleuritis, peritonitis with pleural effusion, thoracic operation, or breast cancer operation on the diseased side), body mass index (BMI), diagnosis, diseased side, preoperative respiratory function measured by spirometry (restrictive disorder, obstructive disorder), chest X-ray findings (blunting of the CP angle), procedure type, CT findings (positive was defined as thickened interlobar shadow), surgical approach (complete VATS vs. thoracotomy), intraoperative blood loss, duration of surgery, duration of chest tube, intrathoracic adhesion (severity, range, characteristics), complications (prolonged air leak defined as an air leak lasting longer than 5 days, arrhythmia, pneumonia, delirium, acute respiratory distress syndrome, chylothorax), and duration of postoperative hospitalization. Complications were defined as any deviations from the normal postoperative course and were recorded according to the Clavien-Dindo classification (23,24). We followed up three months after surgery.
Statistical analysis
A prospective sample size were calculated based on the previous studies (18-22). We expected to include 30% patients with intrathoracic adhesions in this study. Considering a statistical power of 80% and a significant level of 5% (2-sided), the sample size was estimated to be 152 patients. Expecting a dropout rate of 10%, we initially aimed to recruit 167 patients.
For univariate analysis, inter-group differences were evaluated using the non-parametric Wilcoxon rank-sum test. The χ2 or Fisher’s exact test was used to compare categorical variables.
Risk factors included in multivariate analyses were selected on the basis of univariate analysis, statistical independence, and clinical significance. Multivariate logistic regression was used to identify independent risk factors of severe intrathoracic adhesions. Nominal logistic regression was initially performed with variables found to be significant univariate predictors of the outcome being modeled. Because of the rarity of the outcome events being modeled, variables identified in this step with a P-value cutoff of 0.1 were taken as candidates for multivariate logistic regression models.
The prognostic value of intrathoracic severe adhesion was evaluated using receiver operating characteristic (ROC) analysis. For ROC curve analysis, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy were used to predict the outcome in the data set. The method of Hanley et al. was used to calculate the area under curve (AUC) for the index (25,26).
All statistical analyses were performed using JMP version 12.0 (SAS Institute, Inc., Cary, NC, USA). Significance was defined as P<0.05. All reported P-values were two-sided.
Results
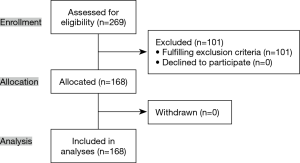
In total, 269 patients were assessed for eligibility, 101 of whom were excluded after the application of exclusion criteria. A total of 168 patients consented to participate and were enrolled. No patient withdrew from the study (Figure 2). Table 1 details the characteristics of the study population. The degree of severe adhesion was divided into hard (such as “stuck”) or soft (such as “spider web”) adhesion, and the former was more prevalent. All 15 patients without sliding lung sign had hard severe adhesions (specificity 100%) (Figure 3A). In this series, the sensitivity, PPV, and NPV of the sliding lung sign were 88.2%, 100%, and 98.7%, respectively. There were two false-negative results, both of which were soft adhesions (Figure 3B).
Full table
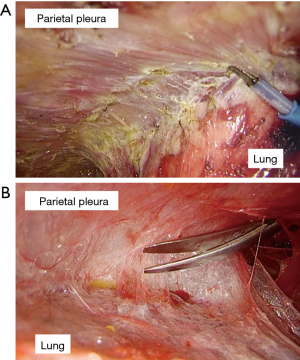
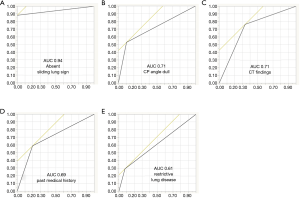
Table 2 details the results of univariate analysis of potential risk factors of severe intrathoracic adhesion and performance characteristics of the absence of sliding lung sign. Older age, absence of the sliding lung sign, blunting of the CP angle, past medical history, CT findings, and restrictive lung disease were identified as significant risk factors of severe intrathoracic adhesion. Figure 4 illustrates the AUC of each factor, with absence of the sliding lung sign having the highest AUC. Because of the significant correlation between the absence of the sliding lung sign and other factors (past medical history, restrictive disorder, blunting of the CP angle, CT findings), multivariate logistic regression analysis was performed using the sliding lung sign as the independent variable. A significant positive correlation was identified between severe intrathoracic adhesion and absence of the sliding lung sign [odds ratio (OR), 8.5; 95% CI, 2.3–31.3; P<0.0001].
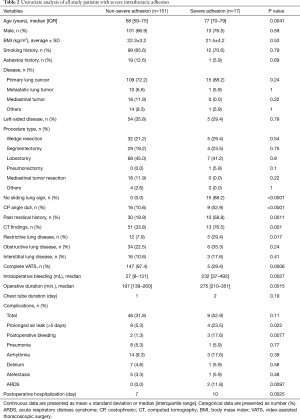
Full table
Thoracotomy was significantly more frequent among patients with severe adhesions [12 patients (70.6%) vs. 4 patients (2.6%); P=0.0006], and intraoperative bleeding, operative time, and postoperative hospitalization were significantly more common in these patients than in those with non-severe adhesions. Duration of chest tube was not significantly different between the groups (Table 2).
Discussion
The sliding lung sign is a sonographic finding that reflects the back-and-forth sliding of the visceral and parietal pleura past one another during respiration. The present study demonstrates that severe intrathoracic adhesions can be predicted prior to surgery through the absence of the sliding lung sign. We identified various empirical factors that may suggest intrathoracic adhesions, including history of thoracic surgery, blunting of the CP angle, CT findings of thickened interlobar shadow, and restrictive lung disorder. Nevertheless, the sliding lung sign was found to be the most valuable and accurate method for the detection of severe intrathoracic adhesions. There was no chest wall invasions, pleural invasions, or pleural dissemination in severe intrathoracic adhesion cases without sliding lung sign, although three patients had pathological T3 lung cancer. There was no obvious causal relationship to the pathological findings.
Predicting severe intrathoracic adhesions before surgery has many advantages. It enables appropriate decision-making with respect to surgical approach, safe installation of the first port, appropriate time-management of the surgery, and assessment of the risk of complications. In the case of emergency surgery, it is possible that the surgeon and staff will be under psychological stress, possibly inhibiting decision-making (27,28). Therefore, it is critically important that all medical staff as well as surgeons and assistants be adequately prepared. If a patient has severe adhesions, the appropriate treatment would be to use a minimal thoracotomy approach. However, if uniportal VATS or robot-assisted thoracoscopic surgery are required, the approach may need to be reconsidered. In the present study, severe intrathoracic adhesions were significantly associated with thoracotomy conversion, more bleeding, longer operative time, increased postoperative complications, and longer postoperative hospital stays.
Previous studies have reported the sliding lung sign to be an effective indicator of tumor chest-wall invasion (7,9,10). Pulmonary respiratory changes can be detected by ultrasonography, as well as by MRI (8); however, ultrasonography has the advantages that it is a low-cost approach that involves no radiation exposure; it minimizes distress to the patient in terms of invasive or claustrophobia procedures; it can be carried out anytime, anywhere, and with any number of scans; and it produces data that are easy to evaluate. The understanding of the sliding lung sign is easy once you see it, and everyone can immediately practice and evaluate it quite precisely (29). However, evaluation of adhesions of the hilum, mediastinum, and apex are difficult using ultrasonography. For these sites, cine MRI may be more suitable. Vascular sheath adhesions, by contrast, may be difficult to evaluate using any modality.
We analyzed two cases of severe intrathoracic adhesion that exhibited the sliding lung sign. These were both cases of soft adhesion, and therefore ultrasonography did not detect adhesions; CT findings, presence of restrictive lung disorder, and past medical history were also negative. Two cases were not peripheral tumor, and clinical and pathological T1b. The pathology-related adhesions was considered negative. However, blunting of the CP angle was observed in both, suggesting that this may be used to detect soft adhesions that cannot be detected by the ultrasonography, thereby compensating for the limitations of ultrasound.
In the field of abdominal surgery, the prevention of adhesion has been a key area of research and development (1-3). In thoracic surgery, aspirin has been reported to reduce postoperative pleural adhesions by inhibiting the expression of platelet-derived growth factor (30). However, prolonged air leaks are a common complication of thoracic surgery. Intrathoracic adhesions have been reported to effectively prevent prolonged air leaks, and pneumothorax can be cured or prevented from recurring using pleurodesis (18). The development of a material that can create specific adhesions of the visceral pleura without any restrictive disorders would be beneficial to the field.
Limitations
The present study has several limitations that should be acknowledged. As a single-center study with a limited number of consecutive cases, the generalizability of the results may be limited. Furthermore, the definition of severe intrathoracic adhesion represents a limitation because the operative duration may be affected by the particular surgeon or staff team. However, the chief surgeon participated in all surgeries and controlled them. Furthermore, data of past medical history may not be accurate because of the interview-style data collection. Nevertheless, this was a prospective study, and all surgical and echography procedures were overseen by the chief surgeon.
Conclusions
The ultrasound sliding lung sign could be used to detect severe intrathoracic adhesions prior to surgery. Preoperative evaluation of ultrasound data may therefore inform clinical decision-making with respect to appropriate surgical plans. Ultrasound examination may be insufficient for the detection of soft adhesions; in these cases, evaluation of the CP angle may provide useful complemented information. These findings suggest that both the ultrasound sliding lung sign and the CP angle should be confirmed prior to surgical decision-making.
Acknowledgments
This study was presented at the 24th European Conference on General Thoracic Surgery of the European Society of Thoracic Surgeon by Takahiro Homma.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-886
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-886
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-886). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study and all its protocols were approved by the Ethics Committee of the Joetsu General Hospital (J-70). All patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- ten Broek RP, Bakkum EA, Laarhoven CJ, et al. Epidemiology and prevention of postsurgical adhesions revisited. Ann Surg 2016;263:12-9. [Crossref] [PubMed]
- Han ES, Scheib SA, Ptzkowsky KE, et al. The sticky business of adhesion prevention in minimally invasive gynecologic surgery. Curr Opin Obstet Gynecol 2017;29:266-75. [Crossref] [PubMed]
- van Steensel S, van den Hil LCL, Schreinemacher MHF, et al. Adhesion awareness in 2016: An update of the national survey of surgeons. PLoS One 2018;13:e0202418. [Crossref] [PubMed]
- Park AE, Zahiri HR, Hallbeck MS, et al. Intraoperative “micro breaks” with targeted stretching enhance surgeon physical function and mental focus: a multicenter cohort study. Ann Surg 2017;265:340-6. [Crossref] [PubMed]
- Weigl M, Stefan P, Abhari K, et al. Intra-operative disruptions, surgeon’s mental workload, and technical performance in a full-scale simulated procedure. Surg Endosc 2016;30:559-66. [Crossref] [PubMed]
- Sonoda Y, Onozuka D, Hagihara A. Factors related to teamwork performance and stress of operating room nurses. J Nurs Manag 2018;26:66-73. [Crossref] [PubMed]
- Sugama Y, Tamaki S, Kitamura S, et al. Ultrasonographic evaluation of pleural and chest wall invasion of lung cancer. Chest 1988;93:275-9. [Crossref] [PubMed]
- Kajiwara N, Akata S, Uchida O, et al. Cine MRI enables better therapeutic planning than CT in cases of possible lung cancer chest wall invasion Lung Cancer 2010;69:203-8. [Crossref] [PubMed]
- Bandi V, Lunn W, Ernst A, et al. Ultrasound vs. CT in detecting chest wall invasion by tumor: a prospective study. Chest 2008;133:881-6. [Crossref] [PubMed]
- Tahiri M, Khereba M, Thiffault V, et al. Preoperative assessment of chest wall invasion in non-small cell lung cancer using surgeon performed ultrasound. Ann Thorac Surg 2014;98:984-9. [Crossref] [PubMed]
- Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest 1995;108:1345-8. [Crossref] [PubMed]
- Joyner CR Jr, Herman RJ, Reid JM. Reflected ultrasound in the detection and localization of pleural effusion. JAMA 1967;200:399-402. [Crossref] [PubMed]
- Sandweiss DA, Hanson JC, Gosink BB, et al. Ultrasound in diagnosis, localization, and treatment of loculated pleural empyema. Ann Intern Med 1975;82:50-3. [Crossref] [PubMed]
- Wang Y, Gargani L, Barskova T, et al. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease:a literature review. Arthritis Res Ther 2017;19:206. [Crossref] [PubMed]
- Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008;134:117-25. [Crossref] [PubMed]
- Chandrasekhar AJ, Reynes CJ, Churchill JC. Utrasonicially guided percutaneous biopsy of peripheral pulmonary masses. Chest 1976;70:627-30. [Crossref] [PubMed]
- Weaver B, Lyon M, Blaivas M. Confirmation of endotracheal tube placement after intubation using the ultrasound lung sign. Acad Emerg Med 2006;13:239-44. [Crossref] [PubMed]
- Leo F, Dellamonica J, Venissac N, et al. Can chest ultrasonography assess pleurodesis after VATS for spontaneous pneumothorax? Eur J Cardiothorac Surg 2005;28:47-9. [Crossref] [PubMed]
- Eshraghi M, Kachoie A, Sharifimoghadam S. Ultrasonography in the diagnosis of lung adhesion before surgery. Biomol Concepts 2019;10:128-32. [Crossref] [PubMed]
- Marchetti G, Valsecchi A, Indellicati D, et al. Ultrasound-guided medical thoracoscopy in the absence of pleural effusion. Chest 2015;147:1008-12. [Crossref] [PubMed]
- Tateishi U, Morikawa T, Miyasaka K. Detection of pleural adhesions with sonography. J Clin Ultrasound 2001;29:61-2. [Crossref] [PubMed]
- Sasaki M, Kawabe M, Hirai S, et al. Preoperative detection of pleural adhesions by chest ultrasonography. Ann Thorac Surg 2005;80:439-42. [Crossref] [PubMed]
- Seely AJE, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Hajian-Tilaki K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J Intern Med 2013;4:627-35. [PubMed]
- Hanley JA. Receiver operating charactersitic (ROC) methodology: the state of the art. Crit Rev Diagn Imaging 1989;29:307-35. [PubMed]
- Dias RD, Scalabrini Neto A. Acute stress in residents during emergency care: a study of personal and situational factors. Stress 2017;20:241-8. [Crossref] [PubMed]
- Aisling M, Aisling D, David C. An assessment of psychological need in emergency medical staff in the northern health and social care trust area. Ulster Med J 2016;85:92-8. [PubMed]
- Knudtson JL, Dort JM, Helmer SD, Smith RS. Surgeon-performed ultrasound for pneumothorax in the trauma suite. J Trauma 2004;56:527-30. [Crossref] [PubMed]
- Ishihara S, Ito K, Okada S, et al. Suppressive effects of aspirin for postthoracotomy pleural adhesion in rats. Int J Med Sci 2019;16:593-601. [Crossref] [PubMed]