Robotic lobectomy and segmentectomy for lung cancer: results and operating technique
Introduction
The paradigm shift—encapsulated by the phrase ‘from maximum tolerated treatment to minimum effective treatment’—that has involved many areas of surgical oncology, has scarcely touched thoracic surgery. Although minimally invasive techniques like video-assisted thoracic surgery (VATS) and robot-assisted surgery, that avoid division of major thoracic muscles and rib-spreading, are available for resecting lung tumours, they are not widely used. A survey conducted by the European Society of Thoracic Surgeons in 2007 found that only around 5% of responding European surgeons were using VATS for pulmonary resections (1). This, notwithstanding the fact that a systematic review of VATS in comparison to thoracotomy for early-stage non-small cell lung cancer (NSCLC)—which included randomized controlled trials—found that VATS was associated with shorter chest tube duration, shorter length of hospital stay, and better survival (at 4 years) than open surgery, all differences being statistically significant (2). Other data show that VATS is associated with reduced postoperative pain, reduced need for blood transfusions and reduced postoperative complications, as well as improved aesthetic and functional outcomes leading to better quality of life (QOL) (3).
The most frequent reason given by surgeons for not using VATS for lobectomy was that it was a difficult technique with a steep learning curve (1).
It would appear that VATS has drawbacks that made its widespread adoption by thoracic surgeons slow. These include counter-intuitive hand movements to manipulate the instruments, an instrument fulcrum effect, and tremor amplification. The surgeon stands over the patient to operate the instruments, while the virtual operating field is displayed on a monitor some distance away, disrupting eye-hand coordination. Furthermore most VATS endoscopes provide low-definition 2-dimensional images with limited magnification possibilities. VATS systems are therefore characterized overall by poor ergonomics, making delicate manoeuvres difficult.
Robotic surgery was introduced at the end of 1990s in part to overcome the limitations of minimally invasive surgery. Probably the first series using a robotic system to perform lung lobectomy was published in 2002 (4). The only currently available robotic systems for performing thoracic surgery are da Vinci Systems produced by Intuitive Surgical, Sunnyvale, California.
The main advantages of robotic technology over VATS are that natural movements of the surgeon’s hands and wrists are translated by the computer-assisted robotic arms into precise movements of the surgical instruments inside the patient, with tremor filtration. The surgeon works at a console some distance from the patient and views the operating field in the console monitor, so that the eye-hand-operating field axis is maintained. The endoscope, directly manipulated by the surgeon at the console, feeds variable magnification, high-definition stereoscopic images to the monitor, which may compensate for the absence of haptic feedback (5).
However these are theoretical advantages, and if the trend to less aggressive oncological surgery is also to involve the thorax, then robotic surgery must be shown to be easier than VATS, and produce equivalent or better surgical and oncological outcomes. Furthermore the high capital and running costs of robot systems (6) will need to be reduced, and opportunities for training or retraining thoracic surgeons will need to be expanded.
Robotic lobectomy—published experience
Lobectomy with lymph node dissection is standard of care for stage I and II NSCLC (7). Following the initial reports (4), the feasibility and safety of robotic lung lobectomy was investigated in a series of studies published over in the subsequent 10 years. Park et al. (8) reported on 34 cancer lobectomies using a three robotic-arm technique (two thoracoscopic ports and a 4-cm utility incision without rib spreading) in which patient and port positions were similar to those used in VATS, and the surgical steps reproduced those of VATS lobectomy, with anterior-to-posterior hilum isolation. Four patients were converted to thoracotomy. A median of 4 (range, 2-7) lymph node stations was removed. There were no perioperative deaths. Median chest tube duration was 3.0 days (range, 2-12 days), median length of stay was 4.5 days (range, 2-14 days) and median operating time was 218 minutes (range, 155-350 minutes). Gharagozloo et al. 2009 (9) reported on 100 consecutive cases operated on with a hybrid two-phase procedure: robotic vascular, hilar and mediastinal dissection, followed by VATS lobectomy. The complication rate was 21% and three patients died postoperatively, considered due to the inclusion of high risk cases. There were no deaths among the last 80 cases, and the first 20 patients were considered to represent the learning phase. The authors considered that the robotic system was best for fine dissection (lymphadenectomy) while the established VATS procedure was superior for the lobectomy phase.
Veronesi et al. (5) 2010 presented the first comparison of open muscle-sparing thoracotomy with robotic lobectomy using a four-arm technique and 3-4 cm access port. Propensity scores for preoperative variables were used to match the 54 robotic cases with 54 patients who received open surgery. Hospital stay was shorter in the robotic group, but operating times were longer; however after the first tertile of cases, the duration of surgery reduced significantly. The authors concluded that robotic lobectomy with lymph node dissection was practicable and safe. The mean duration of the robotic lobectomy was around 220 minutes for the initial cases and around 170 minutes during the last phase of the experience (data not presented).
Dylewski et al. 2011 (10) reported on 200 lung robotic resections using an approach in which pulmonary resection was performed through the ports only, and pneumothorax was induced by CO2 insufflation. At the end of the procedure the specimen was extracted via a subcostal trans-diaphragmatic approach, and the diaphragm subsequently repaired. Median duration of surgery was short at 100 minutes (range, 30-279 minutes) and median hospital stay was three days. However, the readmission rate was high (10%) usually for effusion, requiring drainage, or postoperative pneumothorax.
Like Veronesi et al. (5) 2010, Cerfolio et al. 2011 (11) used a propensity score to match 106 consecutive patients who received robotic lobectomy to 318 patients who received open rib and nerve-sparing lobectomy. The robotic group had numerically lower morbidity and mortality (0% vs. 3.1%), significantly better mental QOL and significantly shorter hospital stay (2.0 vs. 4.0 days). However operating time was significantly longer with the robotic approach (2.2 vs. 1.5 hours). During their experience, the authors modified their technique to add a fourth robotic arm, a vessel loop to guide the stapler, CO2 insufflation, and specimen removal though a supra-diaphragmatic 15 mm access port—changes which reduced operating times and conversions. Cases with larger tumours, hilar node involvement, or previous chemoradiation for nodal involvement were not excluded, amounting to enlarged indications for minimally invasive lung cancer resection. The authors commented that the robot made it possible to perform an “outstanding” node dissection.
Schmid’s group in Innsbruck (12) in 2011 compared posterior (first five patients) and anterior robotic techniques in a learning series of 26 patients. Median hospital stay was 11 days (range, 7-53 days), median operating time was 228 min (range, 162-375 min), and one death occurred within 30 days. The group initially favoured the robotic technique, but in a review stated (13) that they had returned to VATS for major lung resection as the clinical advantages of the robotic approach were insufficient to justify the greater expense and longer operating times.
In 2012 Louie et al. (14) published a case-control evaluation of 53 consecutive robotic lobectomies or segmentectomies and 35 anatomic VATS resections, with nodal stations sampled in both groups. Although surgical and postoperative outcomes were similar in the two groups, robotic cases had significantly shorter duration of narcotic use and earlier return to normal activities. The authors reported that the two approaches afforded similar possibilities for performing mediastinal lymph node dissection; however robotics gave greater confidence in dissecting hilar lymph nodes.
The publication of Park et al. (15) is the only one so far to evaluate long-term oncological outcomes after robotic lobectomy. This study examined 325 consecutive patients who underwent robotic lobectomy for NSCLC at three centres (two in Italy, one in the USA) between 2002 and 2010. Most (76%) cancers were stage I, 18% were stage II, and 6% were stage III. Median follow-up was 27 months. Overall 5-year survival was estimated at 80% [95% confidence interval (CI): 73-88%]: 91% (95% CI: 83-99%) for stage IA, 88% (95% CI: 77-98%) for stage IB, and 49% (95% CI: 24-74%) for stage II. For stage IIIA patients, 3-year survival was 43% (95% CI: 16-69%). These findings suggest that robotic lobectomy for NSCLC affords long-term stage-specific survival consistent with historical results for VATS and thoracotomy.
The number of lymph nodes removed was used as an indirect indicator of oncological radicality in the comparative studies of Veronesi et al. (5) and Cerfolio et al. (11). Median numbers of lymph nodes removed were indistinguishable in the robotic and open procedures, suggesting that the robotic approach achieves similar oncological radicality to that achieved by thoracotomy. Two other studies (14,16) found no differences in numbers of lymph nodes removed by VATS and robotic lobectomy for lung cancer.
The frequency of nodal metastases identified in clinically node-negative cases is another indirect indicator of oncological radically. The paper by Park et al. (15) on 325 robotic lobectomies found that 13% of stage I cases were upstaged to N1. This is similar to upstaging rates reported after open surgery by Boffa et al. 2012 (17) and higher than VATS (18) suggesting that robotic surgery may offer better radicality than VATS. Wilson et al. (19) retrospectively reviewed patients with clinical stage I NSCLC after robotic lobectomy or segmentectomy at three centres. They found the overall rate of pathologic nodal upstaging of 10.9%, 6.6% for hilar (pN1) upstaging and 4.3% for mediastinal (pN2) upstaging. After comparing their findings to those for VATS and open thoracotomy as reported in recent publications (2,17,18,20) and adjusting for clinical T stage according to the AJCC, 7th edition, the authors concluded that rate of robotic pathologic nodal upstaging for clinical stage I NSCLC was superior to that for VATS and similar to that for thoracotomy.
Park et al. (21) reported that the initial capital cost of the da Vinci robot system was about a million USD in 2008, annual maintenance was 100,000 USD, and cost of disposables 730 USD per operation. They estimated that it was about 3,981 USD more expensive to use per operation than VATS. Nevertheless the robotic operation was cheaper than open thoracotomy (by about 4,000 USD), mainly because thoracotomy patients remained in hospital longer.
The costs of using a robotic system for lobectomy and wedge resection were evaluated in a recent study by Swanson et al. (22) in which records of 15,502 lung surgery cases from the Premier hospital database were analysed. Only 4% of surgeries were robot assisted and a propensity score was used to create well matched groups for analysis. Using robotic assistance was associated with higher average hospital costs per patient: lobectomy, USD 25,040.70 for robotic vs. USD 20,476.60 for VATS (P=0.0001); wedge resection USD 19,592.40 vs. USD 16,600.10 (P=0.0001). The study also found that operating times were longer for both lobectomy (robotic 4.49 vs. VATS 4.23 hours; P=0.0969) and wedge resection (robotic 3.26 vs. VATS 2.86 hours; P=0.0003). Length of stay was similar with no differences in adverse events. Another recent study Nasir et al. (23) analysed “approximate financial data” for robotic lung operations performed by one North American surgeon (282 lobectomies, 71 segmentectomies, 41 conversions to open). Median hospital charges were USD 32,000 per patient with hospital profit of USD 4,750 profit per patient. Major morbidity occurred in 9.6%, 30-day operative mortality was 0.25%, and 90-day mortality was 0.5%. And median patient reported pain score was 2/10 at examination 3 weeks after discharge. The authors commented that although these costs were high they were still profitable for the hospital.
Cost analysis of the author experience showed a mean total cost for a robotic lobectomy of around 12.000 euros which is covered by the Italian health reimbursement with no net profit or loss for the hospital.
Robotic lobectomy—technique
Techniques for robotic lobectomy vary. The Milan group uses a four-arm system—three robot arm ports and a utility incision (5). Other authors (4,8) in New York and Pisa started out using three arms, but later adopted a four-arm technique. Dilewski et al. (10) and Cerfolio et al. (11) use a four-arm technique but making a utility incision only at the end of the procedure because they insufflate the chest cavity with CO2 to facilitate access. The position of the utility incision (mainly to remove the surgical specimen) varies with surgeon preference. Veronesi and Park use a fourth intercostal space incision, Dylewski et al. 2011 (10) use a subcostal 2-4 cm trans-diaphragmatic incision, and Cerfolio et al. (11) an incision between ribs 9 and 10 that can be used to extract large tumours. Gharagozloo et al. (9) use a hybrid robotic-VATS technique.
Preoperative assessment and indications
Indications for robotic lobectomy do not differ from those for VATS lobectomy. Patients must have adequate cardiopulmonary reserve, and lesions that are resectable by lobectomy or segmentectomy. Some surgeons (10,11) are using robotic lobectomy on patients with advanced lung cancer after induction treatment, lymph node involvement, and centrally located lesions that require bronchial sleeve resection, which apparently satisfactory results. Standard staging is performed and includes CT with contrast (chest, brain upper, abdomen), and CT/PET (positron emission tomography). For centrally-located lesions bronchoscopy is performed. CT-guided biopsy is performed when a preoperative diagnosis is necessary, for example in patients with co-morbidities, for lesions not highly suspiciousness for cancer, and for centrally located lesions that cannot be removed by (VATS) wedge resection.
Patient positioning and port placement
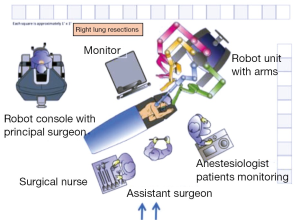
The patient is positioned in lateral decubitus and single-lung anaesthesia induced via a double lumen endotracheal tube. The robot is positioned slightly behind the patient’s head (Figure 1).
Using the four-arm technique, three port incisions and a utility incision are made. First entry (if VATS wedge resection not performed, see below) is via a 1 cm incision through the eighth intercostal space at the level of the mid-axillary line, A 30-degree stereoscopic camera is inserted to explore the thoracic cavity and provide visual guidance for the successive 3 cm utility incision, which is made through the fourth or fifth intercostal space anteriorly (Figure 2). This is followed by an 8-mm incision at the eighth intercostal space in the posterior axillary line for the right robotic arm (on the right side), and another incision in the auscultatory triangle posterior, for the final robotic arm. This fourth incision makes it possible to retract the lung and better expose the operating field.
The ports are standard for all lobectomies except that, on the right side, the camera port through the seventh intercostal space is in the mid axillary line, whereas on the left side this port is moved 2 cm posteriorly (compared to the right) to avoid the heart obscuring vision of hilar structures.
Lesions without a preoperative diagnosis are first excised by standard VATS wedge resection followed by intraoperative frozen section examination.
Small or deep undiagnosed lesions can be located by injecting a solution containing 99Tc-labeled colloid and radio-opaque (iodinated) tracer into the nodule under CT control not more than 24 hours before surgery (24). During surgery a gamma ray-detecting probe is introduced through a port to precisely locate the ‘hot’ nodule and guide the wedge resection.
The lobectomy commences by isolating hilar elements using a hook or spatula and two Cadière graspers. The hook is manipulated by the right arm of the robot introduced through the utility thoracotomy for right side dissections or through the posterior trocar in the eighth intercostal space for left side lobectomies. One of the Cadière graspers (fourth robotic arm) is used to retract the lung and expose structures. The other grasper is manipulated by the left arm of the robot and used to grip structures during dissection. When a hilar vessel or bronchus is ready to be surrounded with a vessel loop for stapler introduction, a third grasper is introduced (substituting the hook). Vessels and the bronchus are sectioned using mechanical staplers introduced through a thoracoscopic port by the assistant surgeon after removal of a robotic arm. The pulmonary vein is usually the first structure to be isolated and divided. If the lesion is in the right upper lobe, vein resection is followed by isolation of the branches of the pulmonary artery and sectioning, followed by isolation of the bronchus and bronchus sectioning. If the lesion is in the right lower lobe or left lung, after pulmonary vein sectioning, the bronchus is usually isolated and stapled before the artery. When performing middle lobectomy, the most favourable sequence is vein, bronchus and artery.
The incomplete fissure is usually prepared with an Endo GIA Autosuture stapler (Coviden) introduced by the assistant surgeon through one of the ports. The lobe is extracted through the anterior utility thoracotomy in an Endo Catch (Covidien) pouch.
Lymph node dissection
While suspicious lymph nodes are usually removed before lobectomy, radical lymph node dissection is performed after lobectomy using the same technique as in open surgery. Para-tracheal lymph node dissection is performed on the right side without azygos vein division. The mediastinal pleura between the superior vena cava and the azygos vein are incised. The lymph nodes, together with the fatty soft tissue of the region of the Barety space, are removed en bloc using a hook and Cadière grasper. In patients with large quantities of mediastinal fat or very large lymph nodes an UltraCision harmonic scalpel (Ethicon) may be used.
The nodes of the subcarinal station are removed after resection of the pulmonary ligament and retraction of the lung towards the anterior mediastinum to expose the posterior mediastinum. Bronchial arteries can usually be avoided thanks to good visibility, if not they are simply coagulated; a clip is not usually required. Tachoseal is sometimes applied to the fissure surface to reduce air leakage. A single 28 Ch (Tyco Healthcare) pleural drain is positioned at the end of the operation.
Segmentectomy
Anatomic segmentectomy is excision of one or more bronchopulmonary segments, with ligation and division of the bronchi and vessels serving those segments. Usually bronchial, hilar, and mediastinal vascular lymph nodes are examined intraoperatively and only patients with N0 disease receive segmentectomy; others receive lobectomy (25). Segmentectomy and also wedge resection—removal of a small wedge-shaped portion of the lung without intraoperative examination of sampled nodes—have been viewed as mainly suitable for elderly patients or those with impaired lung function, who cannot tolerate lobectomy (26), particularly since the publication of a randomized trial comparing sublobar resection (segmentectomy or wedge resection) with lobectomy in patients with T1-2N0 NSCLC, able to tolerate lobectomy (26,27). After a minimum follow-up of 4.5 years, the trial survival was non-significantly worse, and there were more recurrences (significant) in the sublobar resection arm; however failure seemed to mainly occur in patients who received wedge resection (26,27). By contrast non-randomized studies have reported similar survival rates for segmentectomy and lobectomy (28-30). Furthermore a 2014 meta-analysis (31) which examined overall survival and disease-free survival in patients who underwent sublobar resection and were eligible for lobectomy, found that long-term survival was similar for sublobar resection and lobectomy patients.
Interest in performing segmentectomy has grown since the results of the randomized National Lung Screening Trial (NLST) were published in 2011. This trial, which enrolled 53,000 high-risk North American smokers over 55 years of age, found that mortality was reduced by 20% in the low dose CT-screened arm compared to the arm screened by chest radiography (32).
As result of this study lung cancer screening is becoming more widely adopted (33) and small early-stage lesions cancers will constitute an increasing proportion of lung cancers diagnosed. It is likely that many of these small cancers will be adequately treated by segmentectomy or wedge resection which could ideally be performed using minimally invasive robot-assisted surgery. A number of ongoing trials are now re-examining the role of sub-lobar resection for small early-stage lung cancer.
The Cancer and Lymphoma Group B (CALGB 140503) is conducting a prospective, randomized multi-institutional phase III trial to determine whether sublobar resection is non-inferior, in terms of survival and recurrence, to lobectomy in patients with a small (≤2 cm) single peripheral lesion, confirmed as stage IA NSCLC (34). The trial aims to recruit about 1,300 patients.
Another randomized phase III non-inferiority trial is being conducted in Japan (35). Patients with a single peripheral stage IA NSCLC lesion ≤2 cm are randomized to segmentectomy/wedge resection vs. lobectomy. The trial aims to recruit 1,100 patients from 71 institutions over 3 years.
A Milan is co-coordinating an Italian multicentric phase III randomized trial comparing sublobar resection to standard lobectomy. The aim is to recruit 810 patients over 3 years. Eligibility criteria are similar to those of the trials cited above. However there will also be preoperative stratification with CT-PET to identify a subgroup who are PET-negative, have a lesion ≤1 cm, or both. Eligibility criteria are checked intraoperatively and if satisfied patients are randomized. For patients in the PET-negative/≤1 cm subgroup, lymph node sampling is not performed before randomization and if randomized to segmentectomy/wedge resection, receive only lung resection. Patients randomized to lobectomy receive both lobectomy and lymphadenectomy. Patients with nodule >1 cm and positive at PET receive lymph node sampling with preoperative frozen section: only those with a negative frozen section at three lymph node levels and negative margin at resection line are randomized.
Robotic segmentectomy—published experience
Few papers on robotic pulmonary segmentectomy have been published. The first appears to be a multicentric study involving groups in Milan, the Memorial Sloan Kettering Cancer Center, New York, and Hackensack University Medical Center, New Jersey (36,37). The study reported on 17 patients (7 men, 10 women), mean age 68.2 years (range, 32-82 years) who underwent robotic pulmonary segmentectomy from 2008 to 2010. Mean operating time was 189 minutes (range, 138-240 minutes). Median postoperative stay was 5 days (range, 2-14 days). There were no conversions to VATS or thoracotomy and no postoperative deaths. Early postoperative complications consisted of one (5.9%) case of pneumonia and two (11.9%) cases—both with emphysema—of prolonged air leak. Most cancers (64.7%) were in a lower lobe. Median tumour size was 1.11 cm (range, 0.6-2.8 cm) with NSCLC in 8, typical carcinoid in 2, and lung metastases in 7. In three patients the metastases appeared to be from colon cancer, and in one case each were compatible with breast cancer, adenoid carcinoma, gastrointestinal trophoblastic tumour, and osteogenic sarcoma. Six of the primary lung cancers were pN0, and two were pN1. This initial experience was considered encouraging because it offered all the advantages of minimally invasive surgery plus those inherent in the robotic system. In particular, it proved easy to perform radical dissection of the mediastinal and hilar lymph nodes, with no major bleeding, chylothorax or recurrent laryngeal nerve injury. By contrast, lymphadenectomy with VATS can be challenging (38).
The 2014 paper of Toker et al. (39) reported on 21 patients (15 with malignant disease) who underwent robotic pulmonary segmentectomy using the da Vinci System. There were no conversions. Four patents had postoperative complications. Mean operating time (at the robotic console) was 84 minutes [standard deviation (SD) 26, range, 40-150 minutes]. Mean duration of chest tube drainage was 3 days (SD 2.1, range, 1-10 days) and mean postoperative hospital stay was 4 days (SD 1.4, range, 2-7 days). The authors removed a mean of 14.3 (range, 2-21) nodes from mediastinal stations, and 8.1 (range, 2-19) nodes from hilar and interlobar stations. They concluded, with the previously cited study, that that robot-assisted thoracoscopic segmentectomy for malignant and benign lesions was practical, safe, and associated with few complications and short postoperative hospitalization. They noted that the number of lymph nodes removed appeared “oncologically acceptable” for early lung cancer patients, and that to evaluate postoperative pain, respiratory function and QOL, a prospective comparison with VATS was necessary.
During robotic segmentectomy, it can be challenging to identify intersegmental planes. A new technique to identify these planes has been recently described (40). After division, within the hilum, of the bronchus, vein, and artery of the target segment, the non-toxic fluorescent dye indocyanine green (ICG) is introduced through the peripheral vein catheter, and the robot visual system is changed to fluorescence mode. Mediastinal and parenchymal tissue appears green 30-40 seconds after infusion. The coloration reaches maximum intensity after about a minute and fades slowly. Thus, perfused lung parenchyma becomes green, while the isolated segment (to be removed) remains un-coloured, affording excellent demarcation of the segment and facilitating transection along intersegmental planes with endoscopic staplers. Since lung palpation is not possible with the robotic technique, the clear view of intersegmental planes that ICG affords makes it easier to ensure adequate distance between the lesion and the resection margin. This procedure has so far been used on few patients but appears promising.
Robotic segmentectomy—technique
Principle of anesthesia, patient position and room set up are similar to those or lobectomy.
The position and number of ports is the same as robotic lobectomy described above, and port placement does not vary with side or type of segmentectomy. The isolation of segmental elements is usually performed using a Cadiere and a hook cautery. The ligation of the vascular branches is either performed with an endovascular stapler or between Hem-o-Lok clips (Teleflex Medical, Research Triangle Park, NC). The parenchima is divided with multiple firings of the endoscopic stapler. Lymph node dissection and postoperative care follow the principles of lobectomy.
Conclusions
Randomised studies comparing vats versus robotic approach are not available so far and few papers describe a long term results after robotic resection for lung cancer. The experiences described in the literature confirm that robotic approach is a good and safe alternative to videothoracoscopic approach, and is considered an easier and more intuitive procedure to afford difficult cases, or anatomical segmentectomy. The improved view and intuitive movements seem to favor an increased radicality in locally advanced disease at mediastinal level.
The paradigm shift—encapsulated by the phrase “from maximum tolerated treatment to minimum effective treatment”—hat has involved many areas of surgical oncology, may now also be widely adopted by thoracic surgeons.
Main limitation of robotic procedures is still represented by higher costs of the technique compared to vats as a single company is on the market thus no competition able to reduce prices is possible.
Acknowledgements
Thanks Don for English editing, Raffella Bertolotti for her help as datamanager.
Disclosure: I’ve been proctor in robotic thoracic surgery for Abi medicar.
References
- Rocco G, Internullo E, Cassivi SD, et al. The variability of practice in minimally invasive thoracic surgery for pulmonary resections. Thorac Surg Clin 2008;18:235-47. [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8.
- Cheng D, Downey RJ, Kernstine K, et al. Video-assisted thoracic surgery in lung cancer resection: a meta-analysis and systematic review of controlled trials. Innovations (Phila) 2007;2:261-92. [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [PubMed]
- Crinò L, Weder W, van Meerbeeck J, et al. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v103-15. [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [PubMed]
- Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. [PubMed]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [PubMed]
- Augustin F, Bodner J, Wykypiel H, et al. Initial experience with robotic lung lobectomy: report of two different approaches. Surg Endosc 2011;25:108-13. [PubMed]
- Bodner J, Schmid T, Wykypiel H, et al. Robotic surgery in thoracic cancer. Memo 2010;3:103-5.
- Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598-604; discussion 1604-5. [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [PubMed]
- Jang HJ, Lee HS, Park SY, et al. Comparison of the early robot-assisted lobectomy experience to video-assisted thoracic surgery lobectomy for lung cancer: a single-institution case series matching study. Innovations (Phila) 2011;6:305-10. [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53; discussion 353. [PubMed]
- Licht PB, Jørgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg 2013;96:943-9; discussion 949-50. [PubMed]
- Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901-6; discussion 1906-7.
- Merritt RE, Hoang CD, Shrager JB. Lymph node evaluation achieved by open lobectomy compared with thoracoscopic lobectomy for N0 lung cancer. Ann Thorac Surg 2013;96:1171-7. [PubMed]
- Park BJ, Flores RM. Cost comparison of robotic, video-assisted thoracic surgery and thoracotomy approaches to pulmonary lobectomy. Thorac Surg Clin 2008;18:297-300. [PubMed]
- Swanson SJ, Miller DL, McKenna RJ Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [PubMed]
- Nasir BS, Bryant AS, Minnich DJ, et al. Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann Thorac Surg 2014;98:203-8; discussion 208-9. [PubMed]
- Bellomi M, Veronesi G, Trifirò G, et al. Computed tomography-guided preoperative radiotracer localization of nonpalpable lung nodules. Ann Thorac Surg 2010;90:1759-64. [PubMed]
- Keenan RJ, Landreneau RJ, Maley RH Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33; discussion 228-33. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [PubMed]
- Detterbeck FC. Lobectomy versus limited resection in T1N0 lung cancer. Ann Thorac Surg 2013;96:742-4. [PubMed]
- Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041-5. [PubMed]
- Koike T, Yamato Y, Yoshiya K, et al. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg 2003;125:924-8. [PubMed]
- Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 2001;71:956-60; discussion 961. [PubMed]
- Cao C, Gupta S, Chandrakumar D, et al. Meta-analysis of intentional sublobar resections versus lobectomy for early stage non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:134-41. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [PubMed]
- Bach PB, Mirkin JN, Oliver TK, et al. The Role of CT Screening for Lung Cancer in Clinical Practice. The Evidence Based Practice Guideline of the American College of Chest Physicians and the American Society for Clinical Oncology, 2012. Available online: http://www.asco.org/quality-guidelines/role-ct-screening-lung-cancer-clinical-practice-evidence-based-practice-guideline. [Epub ahead of print].
- Available online: http://www.cancer.gov/clinicaltrials/search/view?cdrid=555324&version=HealthProfessional
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [PubMed]
- Pardolesi A, Park B, Petrella F, et al. Robotic anatomic segmentectomy of the lung: technical aspects and initial results. Ann Thorac Surg 2012;94:929-34. [PubMed]
- Pardolesi A, Veronesi G. Robot-assisted lung anatomic segmentectomy: technical aspects. Thorac Surg Clin 2014;24:163-8. [PubMed]
- Schuchert MJ, Pettiford BL, Pennathur A, et al. Anatomic segmentectomy for stage I non-small-cell lung cancer: comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg 2009;138:1318-25.e1.
- Toker A, Ayalp K, Uyumaz E, et al. Robotic lung segmentectomy for malignant and benign lesions. J Thorac Dis 2014;6:937-42. [PubMed]
- Pardolesi A, Veronesi G, Solli P, et al. Use of indocyanine green to facilitate intersegmental plane identification during robotic anatomic segmentectomy. J Thorac Cardiovasc Surg 2014;148:737-8. [PubMed]