
Intermediate oncologic outcomes after uniportal video-assisted thoracoscopic thymectomy for early-stage thymoma
Introduction
Thymoma is a slow-growing tumor originating from the epithelial cells of the thymus and remains the most common anterior mediastinal neoplasm found in adults (1). Given the rarity and indolent nature of this neoplasm, significant controversy remains regarding the appropriate surgical approach towards treatment of early stage thymoma (stage I or II) (2-6).
Microscopically margin-negative resection has been well-established as the most important prognostic indicator of overall- and recurrence-free survival (RFS) (4-10). Complete surgical resection of early-stage thymoma has historically resulted in excellent oncologic outcomes, with upwards of 100% reported 5-year survival (2-8). While classically performed through a median sternotomy, video-assisted thoracic surgery (VATS) has been reported to achieve equivalent oncologic outcomes (4-6,10-13). However, given the indolent nature of this tumor, intermediate- and long-term data is still lacking with regards to the VATS approach.
Single-incision, or uniportal, VATS thymectomy has been reported (14-17), however, to date, no data exists regarding intermediate-term oncologic outcomes. Consequently, we retrospectively evaluated the overall survival (OS) and RFS in all patients undergoing single-incision VATS thymectomy for early-stage thymoma at our institution. We present the following article in accordance with the STROBE reporting checklist, available at http://dx.doi.org/10.21037/jtd-20-1370.
Methods
Study population and definitions
Between January 2009 and July 2014, a comprehensive retrospective review of a prospectively collected thoracic surgery database identified all patients who underwent single-incision VATS thymectomy for early stage thymoma. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was conducted with the approval of the Northwell Health Institutional Review Board (October 6, 2017. IRB approval #17-0659), consent was waived.
Clinical staging was based on computed tomography (CT) and/or magnetic resonance imaging (MRI) findings. No patients underwent preoperative biopsies. Final pathological diagnosis was based on histology. Pathological staging was based on the Masaoka staging system (18). Histologic subtype was based on the World Health Organization (WHO) classification system (8,9).
Exclusion criteria included all patients without stage I or II thymoma (e.g., late-stage thymoma (III and IV), thymic hyperplasia, thymic carcinoma etc.). Size, in and of itself, was not an exclusion criterion.
Surgical approach
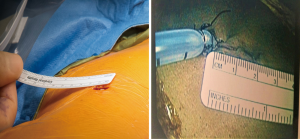
All patients were operated upon by a single surgeon, at a single institution. The preoperative plan was to perform a unilateral single-incision (<3 cm incision; Figure 1) VATS thymectomy, with conversion to open surgery when clinically indicated. All specimens were removed via an endoscopic retrieval bag (which helps to facilitate the removal of large tumors once). Examples of a 5.2 and 9 cm thymoma (Figure 2).

We remove all suspicious lymph nodes based on preoperative imaging. Further, we perform en bloc resection of the thymus, perithymic, prevascular, and supradiaphragmatic lymph nodes. In addition, right para-tracheal and para-aortic lymph nodes are dissected when operating for advanced thymomas and/or thymic carcinomas.
Surgical technique and instruments
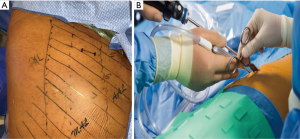
All patients are placed in left lateral decubitus position and secured to the bed, such that they can be placed at a forty-five-degree angle with regards to the floor (our preference is to utilize a bean-bag positioner with silk tape placed across the hip). A single sub-three-centimeter incision is created in the fifth intercostal space between the mid- and anterior-axillary lines (Figure 3A). This incision affords enough space for simultaneous use of multiple instruments (Figure 3B). For larger specimens, the muscular portion of the incision may be extended, however the extent of the skin incision does not need to be enlarged. The operating surgeon positions himself posterior to the patient, whereas the first assistant is subsequently anterior to the patient.
A 5 mm thirty-degree angled thoracoscope provides optimal visualization from phrenic nerve to phrenic nerve. Dissection is carried out utilizing standard VATS instruments (curved and straight ringed forcep, curved lymph node grasper, curved blunt-tip metal suction, harmonic scalpel and endo Kittner). No SILS port or wound-protectors were utilized.
All patients receive multi-level intercostal nerve block, as well as multi-modality post-operative pain control with non-steroidal anti-inflammatory (patient’s creatinine clearance and bleeding are taking under consideration) and narcotic-based, patient-controlled analgesia pump.
Follow up
All patients had short-term follow up, usually within two weeks of discharge from the hospital and then twice per year for the first year. Given the nature of the disease, all patients were encouraged to maintain yearly follow up visits indefinitely (with requisite chest CT imaging prior to follow up visits). If a patient were lost to follow up, the electronic medical records were queried for proof of life. No judgement was made regarding recurrence without chest imaging and a follow-up visit with the thoracic surgeon of record.
Data analysis
Our primary endpoint was OS and secondary endpoint was RFS. Definitions of patient demographic characteristics, perioperative variables, and postoperative outcomes were obtained from the Society of Thoracic Surgeons Adult Thoracic Surgery Database, version 2.81 (http://www.sts.org/registries-research-center/sts-national-database/adult-thoracic-surgery-database/data-collection).
Study data were collected and managed using REDCap electronic data capture tools hosted at Long Island Jewish Medical Center (19). REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: (I) an intuitive interface for validated data entry; (II) audit trails for tracking data manipulation and export procedures; (III) automated export procedures for seamless data downloads to common statistical packages; and (IV) procedures for importing data from external sources.
The OS and RFS curves were calculated using the Kaplan-Meier method.
Results
Patient demographics and operative data
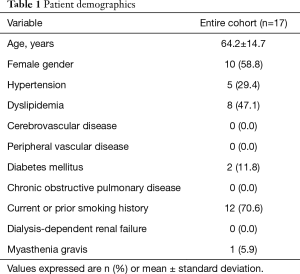
Over the time period of the study, 21 patients underwent elective, first-time, single-incisions VATS thymectomy. Four patients did not meet inclusion criteria: three due to advanced-stage disease (three stage III) and one secondary to a pathologic diagnosis of thymic hyperplasia. Ultimately, seventeen patients underwent resection for early-stage thymoma. There were no documented islands or foci of thymic carcinoma on final pathology. There were ten females and seven males. The average age was 64.2±14.7 and only one patient had diagnosed myasthenia gravis.
Patient demographics are summarized in Table 1.
Full table
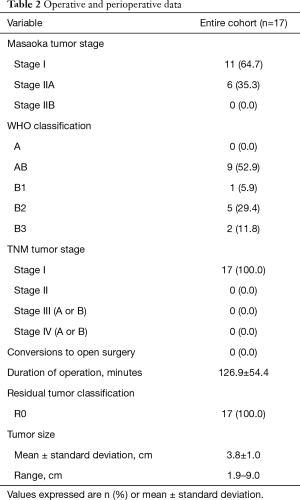
Major intraoperative and perioperative data are reported in Table 2. Eleven of the seventeen patients were classified as stage I and six as stage IIA, according to the Masaoka classification system. The mean surgical duration was 126.9±54.4 minutes. There were no conversions to open surgery. All patients underwent a R0 resection. There were no recorded phrenic nerve injuries in the entire cohort.
Full table
Clinical outcomes
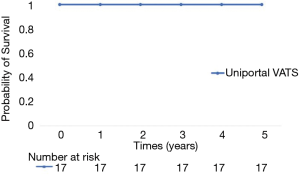
For the entire cohort, 30-day mortality was 0.0% and was without major perioperative surgical complications. The median length of stay for the entire cohort was 1.0 days (range of 1.0–2.0 days), with a median follow-up of 65 months. Overall 5-year survival was 100% (Figure 4), with Kaplan-Meier estimated 5-year RFS of 100% (Figure 5).

Short- and intermediate-term outcomes are summarized in Table 3.

Full table
Discussion
Recent years have seen a trend towards utilizing a VATS approach for treatment of thymoma (20), owing to the well-documented advantages in perioperative outcomes of VATS (6,11-13). While short-term outcomes have shown the VATS approach to be effective (13,21), intermediate- and long-term oncologic data is lacking in larger studies. More recently, large multi-institutional studies from the Japanese Association for Research on the Thymus (JART) (22), the Chinese Alliance for Research in Thymomas (ChART) (23) and the International Thymic Malignancies Group (ITMIG) (24) have sought to address the previously underpowered and short-term follow-up studies.
As it stands, the National Comprehensive Cancer Network does not recommend VATS to be the standard approach for routine resection of thymoma, however, with regards to early-stage (I or II) disease, their stance states “minimally invasive procedures may be considered for clinical stage I–II if all oncologic goals can be met as in standard procedures, and if performed by specialized centers by surgeons with experience in these techniques (25).”
Several reports have shown single-incision VATS thymectomy to be feasible (14-17), however, despite the interest in single-incision thoracic surgery (14-17,26-28), to date, no data exists regarding intermediate-term oncologic outcomes. While technically feasible, critics will argue that given the indolent nature of thymomas, definitive statements should be withheld for up to ten years (1-7). However, as proponents of VATS have long-stated, since the most statistically significant predictor of OS and RFS remains extent of resection (2-6), as long as one adheres to the tenets of oncologic standards, how the neoplasm is removed should be of little consequence. Our data suggests this to be true, as we achieved a R0 resection for the entire cohort, and our 100% 5-year OS compares favorably to the historic standards of Kondo et al. (n=769; 5-year OS: stage I, 100%; stage II, 95%) (29), Murakawa et al. (n=96; 5-year OS: stage I, 91%; stage II, 97%) (30), McCart et al. (n=21; 5-year OS: stage I, 98%%) (31) and Nakahara et al. (n=78; 5-year OS: stage I, 100%; stage II, 92%) (32). Although the aforementioned studies did not report their 5-year RFS, our 100% estimated RFS would compare favorably if they had.
Not surprisingly, our data also compares favorably with VATS studies which have reported 5-year OS: Sakamaki et al. (n=71; 5-year OS: stage I, 94.1%; stage II, 94.7%) (12), Pennathur et al. (n=18; estimated 5-year OS: stage I + II 100%) (33) and Chung et al. (n=24; 5-year OS: stage I+II, 100%) (34). Likewise, our 100% estimated 5-year RFS compares favorably with that of Sakamaki et al. (n=71; 5-year RFS: stage I, 94.1%; stage II, 85.4%) (12), Pennathur et al. (n=18; estimated 5-year RFS: stage I+II 100%) (33) and Chung et al. (n=24; 5-year RFS: stage I+II, 96%) (34).
As shown, single-incision VATS thymectomy for the treatment of early-stage thymoma has favorable intermediate-term oncologic outcomes as compared to the historic standards of median sternotomy and more recent multi-port VATS studies. This data lends support to the feasibility of the single-incision VATS approach for the treatment of early-stage thymoma.
While feasible, there remains no consensus regarding strict contraindications for VATS. Kimura et al. reported that tumors >5 cm substantially increased the risk of capsular rupture and potential seeding of the tumor (35). Alternatively, from their insights from the National Cancer Database, Burt and colleagues did not find a correlation between size and completeness of resection (20). Our data suggest that tumor size is not a prognostic factor with regards to extent of resection, with our entire cohort undergoing a R0 resection. However, while no patients were excluded from undergoing resection based on size, the high end of our range was still 9.0cm. It is our institution’s belief that the ability to perform a sound oncologic procedure, not size, should be the determining factor in choosing a surgical approach.
Limitations
Our study has several limitations to be acknowledged. Despite the fact that our data is comprehensive, our sample size remains relatively small, and our analysis is retrospective and largely observational in nature. Our patient population was highly selected, consisting of those individuals who presented with imaging consistent with non-invasive disease; consequently, our results are not generalizable to patients presenting with clinically more advanced disease, such as stage III or IV thymoma. Given the rarity of this neoplasm, large multi-institutional studies are required, several of which are currently underway. Our length of follow-up can still be considered short, as recurrence has been seen upwards of ten years following initial resection.1-7
Conclusions
In conclusion, our findings suggest that single-incision VATS thymectomy for early-stage thymoma is feasible, and the intermediate-term oncologic outcomes are comparable to historic standards for open and multi-incision VATS thymectomy. However, additional follow-up is required to evaluate for long-term oncologic outcomes.
Acknowledgments
Poster presented at the 57th Annual Meeting Eastern Cardiothoracic Surgical Society. Naples Fl. October 2019.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1370
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1370
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-1370
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1370). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was conducted with the approval of the Northwell Health Institutional Review Board (October 6, 2017. IRB approval #17-0659), consent was waived. There were no human or animal experiments included in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Brambilla E, Mueller-Hermelink HK, et al. WHO classification of tumors. pathology and genetics of tumors of the lung, pleura, thymus and heart. Lyon, France: IARC Press, 2004.
- Nakagawa K, Yokoi K, Nakajima J, et al. Is Thymomectomy Alone Appropriate for Stage I (T1N0M0) Thymoma? Results of a Propensity-Score Analysis. Ann Thorac Surg 2016;101:520-6. [Crossref] [PubMed]
- Ruffini E, Venuta F. Management of thymic tumors: a European perspective. J Thorac Dis 2014;6:S228-37. [PubMed]
- Falkson CB, Bezjak A, Darling G., et al. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol 2009;4:911-9. [Crossref] [PubMed]
- Tomaszek S, Wigle DA, Keshavjee S, et al. Thymomas: review of current clinical practice. Ann Thorac Surg 2009;87:1973-80. [Crossref] [PubMed]
- Girard N, Mornex F, Van Houtte P, et al. Thymoma: a focus on current therapeutic management. J Thorac Oncol 2009;4:119-26. [Crossref] [PubMed]
- Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg. 1996;112:376-84. [Crossref] [PubMed]
- Wright CD, Wain JC, Wong DR, et al. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization histology, and size. J Thorac Cardiovasc Surg 2005;130:1413-21. [Crossref] [PubMed]
- Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer 2002;94:624-32. [Crossref] [PubMed]
- Nakagawa K, Asamura H, Matsuno Y, et al. Thymoma: a clinicopathological study based on the new World Health Organization classification. J Thorac Cardiovasc Surg 2003;126:1134-40. [Crossref] [PubMed]
- Odaka M, Akiba T, Yabe M, et al. Unilateral thoracoscopic subtotal thymectomy for the treatment of stage I and II thymoma. Eur J Cardiothorac Surg 2010;37:824-6. [Crossref] [PubMed]
- Sakamaki Y, Oda T, Kanazawa G, et al. Intermediate-term oncologic outcomes after video-assisted thoracoscopic thymectomy for early-stage thymoma. J Thorac Cardiovasc Surg 2014;148:1230-7.e1. [Crossref] [PubMed]
- Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg 2012;94:974-81. [Crossref] [PubMed]
- Scarci M, Pardolesi A, Solli P. Uniportal video-assisted thoracic surgery thymectomy. Ann Cardiothorac Surg 2015;4:567-70. [PubMed]
- Wu CY, Heish MJ, Wu CF. Single port VATS mediastinal tumour resection: Taiwan experience. Ann Cardiothorac Surg 2016;5:107-11. [Crossref] [PubMed]
- Wu L, Lin L, Liu M, et al. Subxiphoid uniportal thoracoscopic extended thymectomy. J Thorac Dis 2015;7:1658-60. [PubMed]
- Gonzalez-Rivas D, Wu CF, de la Torre M. Uniportal video-assisted thoracoscopic thymectomy and resection of a giant thymoma in a patient witness of Jehova. J Thorac Dis 2017;9:E556-9. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. [Crossref] [PubMed]
- Burt BM, Nguyen D, Groth SS, et al. Utilization of Minimally Invasive Thymectomy and Margin-Negative Resection for Early-Stage Thymoma. Ann Thorac Surg 2019;108:405-11. [Crossref] [PubMed]
- Cheng YJ, Kao EL, Chou SH. Videothoracoscopic resection of stage II thymoma: prospective comparison of the results between thoracoscopy and open methods. Chest 2005;128:3010-2. [Crossref] [PubMed]
- Agatsuma H, Yoshida K, Yoshino I. Video-assisted thoracic surgery versus sternotomy thymectomy in patients with thymoma. Ann Thorac Surg 2017;104:1047-53. [Crossref] [PubMed]
- Gu Z, Chen C, Wang Y. Video-assisted thoracoscopic surgery versus open surgery for stage I thymic epithelial tumours: a propensity score-matched study. Eur J Cardiothorac Surg 2018;54:1037-44. [Crossref] [PubMed]
- Fang W, Xao X, Antonicelli A. Comparison of surgical approach and extent of resection for Masaoka-Koga Stage I and II thymic tumours in Europe, North America and Asia: an international thymic malignancy interest group retrospective database analysis. Eur J Cardiothorac Surg 2017;52:26-32. [Crossref] [PubMed]
- National Comprehensive Cancer Network. 2017 Thymoma and thymic carcinomas. Accessed Sept. 11 2019. Available at https://www.nccn.org/professionals/physician_gls/pdf/thymic.pdf
- Gonzalez-Rivas D, Damico TA, Jiang G, et al. Uniportal video-assisted thoracic surgery: a call for better evidence, not just more evidence. Eur J Cardiothorac Surg 2016;50:416-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Mendez L, et al. Single-port video-assisted thoracoscopic anatomic segmentectomy and right upper lobectomy. Eur J Cardiothorac Surg 2012;42:e169-71. [Crossref] [PubMed]
- Migliore M. Uniportal video-assisted thoracic surgery, and the uni-surgeon: new words for the contemporary world. J Vis Surg 2018;4:45. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84. [Crossref] [PubMed]
- Murakawa T, Nakajima J, Kohno T, et al. Results from surgical treatment for thymoma: 43 years of experience. Jpn J Thorac Cardiovasc Surg 2000;48:89-95. [Crossref] [PubMed]
- McCart JA, Gaspar L, Inculet R, et al. Predictors of survival following surgical resection of thymoma. J Surg Oncol 1993;54:233-8. [Crossref] [PubMed]
- Nakahara K, Ohno K, Hashimoto J, et al. Thymoma: results with complete resection and adjuvant postoperative irradiation in 141 consecutive patients. J Thorac Cardiovasc Surg 1988;95:1041-7. [Crossref] [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. [Crossref] [PubMed]
- Chung JW, Kim HR, Kim DK, et al. Long-term Results of Thoracoscopic Thymectomy for Thymoma without Myasthenia Gravis. J Int Med Res 2012;40:1973-81. [Crossref] [PubMed]
- Kimura T, Inoue M, Kadota Y. The oncological feasibility and limitations of video-assisted thoracoscopic thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2013;44:e214-8. [Crossref] [PubMed]


