Study on risk factors and phenotypes of acute exacerbations of chronic obstructive pulmonary disease in Guangzhou, China—design and baseline characteristics
Introduction
Acute exacerbations are common in chronic obstructive pulmonary disease (COPD) and contribute greatly to reduced quality of life, increased morbidity, frequent emergency department visits, hospital admissions and increased healthcare costs (1-4). Exacerbations further amplify ongoing inflammatory processes in the airways of COPD patients, and may be triggered by environmental pollutants or infection with bacteria or viruses (5,6). The complexity of all those processes needs further exploration, ideally in longitudinal studies. In China, like in many other countries, information is limited about e.g., exacerbation pattern and potential trigger factors, co-morbidities, healthcare utilisation and medical treatment.
The prevalence of COPD in urban and rural populations based clusters from seven provinces/cities in China was investigated by using a standardized questionnaire revised from the international burden of chronic obstructive lung disease (BOLD) study (7). The overall prevalence of COPD defined according to global initiative for chronic obstructive lung disease (GOLD) was 8.2% (men 12.4% and women 5.1%). The prevalence of COPD was significantly higher in: rural residents, elderly patients, smokers, individuals with lower body mass index (BMI), less education, poor ventilation in the kitchen, exposure to occupational dusts or biomass fuels, pulmonary problems in childhood and family history of pulmonary diseases. The rural area with the highest prevalence of COPD was found in the Guangdong province (12%). In another study (8) the prevalence of COPD in non-smokers in a large Chinese population was investigated. An overall prevalence of 5.2% was reported. Although more non-smoking women developed COPD than men a higher risk of developing COPD was associated with male gender, low BMI, low education level, exposure to tobacco smoke, coal, biomass smoke, poor ventilation in the kitchen, a family history of respiratory disease and recurrent childhood cough. In separate studies the influence of passive smoking and the indoor exposure to biomass fuels were indicated as risk factors for the development of COPD in China (9,10). The latter findings explained why around 25% of Chinese females over 70 years of age show an air flow obstruction (11).
Although insight on the prevalence of COPD in China has increased, information on the occurrence and the different trigger factors that lead to exacerbations is still lacking. To address this, a 2-year, single centre, observational hospital-based study in COPD patients in Guangzhou, the capital and largest city of the Guangdong province, was performed. Moreover, the area of Guangzhou is heavily industrialized with a population of around 13 million people. A study design like this one could allow the identification of different trigger factors on exacerbations in a longitudinal way. Moreover this study incorporated a very carefully selected and well documented COPD population and even a COPD population at risk, which is unique for China. This report describes the complete study design, the COPD patient population characteristics at study start and incidence of exacerbations in the cohort.
Methods
Study population
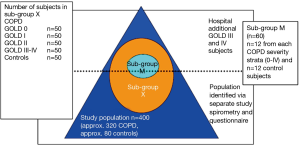
Participants, aged 40-79 years, were stratified into different, but equally sized groups based on severity of COPD according to the GOLD classification. The study aimed to recruit 320 COPD patients and 80 lung-healthy controls. The patients included a group of 80 subjects having a documented medical history in terms of risk factors for COPD and chronic bronchitis without detectable airflow obstruction that suggested the development of COPD (group denoted as GOLD class 0). Age matched healthy volunteers were recruited separately and informed about the study protocol. From the total cohort we aimed to recruit a sub-group X (n=250) to study the occurrence and trigger factors of exacerbations, again with equally sized groups (n=50) according to GOLD stage as well as controls. At enrolment patients for this group were selected on the basis of the following criteria:
- Willingness to be trained to use a Blackberry Personal Data Assistant (PDA) in the forthcoming 2 weeks;
- Willingness to report daily symptom scores with the Blackberry device for 2 years when training had proved ability to use the device as instructed;
- Willingness to pay a visit to the clinic at yearly intervals (visits 1, 2 and 3) and when an exacerbation occurred within 48 hours upon decision of the clinic (extra visits);
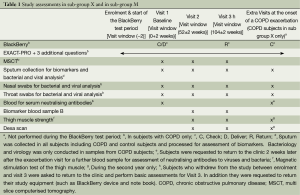
- Willingness to undergo a set of clinical investigations at each visit to the clinic and to donate blood and sputum and throat specimens for further investigation (see Table 1).
Full table
Participants for sub-group X were selected at enrolment on the above mentioned criteria and were trained for 2 weeks to use an electronic diary in a BlackBerry Personal Data Assistant (PDA) (Health Diary Inc. Toronto, Canada) device (see Appendix A) which allowed monitoring of daily symptoms based on 17 questions. When the handheld Blackberry device training was completed successfully they were randomized to one of the GOLD stage groups till n=(50±2) was reached for that group. When the patient did not successfully complete this training the patient was monitored for 2 years without daily symptom score registration. Also when recruitment of the required number for a particular GOLD stage group had been reached the patient was only monitored for 2 years without daily symptom score registration and extra visits to the clinic at an exacerbation.
All daily symptom score information obtained by the Blackberry PDA was transmitted to the study centre immediately after data entry.
At visit 3, after year 1 of study start a subset of Sub-group X, Sub-group M, was selected according to the following criteria:
- Willingness to undergo a set of additional clinical tests: thigh muscle strength and dexa scan (see Table 1);
- All clinical criteria mentioned for sub-group X.
Sub-group M consisted of n= (12±2) COPD patients per GOLD stage group and (12±2) age-matched controls. In sub-group M we aimed to investigate changes in muscle and muscle function during an acerbation (see Figure 1). For more detailed information on the study population, recruitment and inclusion/exclusion criteria, see Appendix B.
Study design
The study was designed as a 2-year, single centre, observational study at the Respiratory hospital of Guangzhou, China. Ethical approval for the study was obtained by the Ethical Committee of the Respiratory hospital of Guangzhou. Participants signed an informed consent form (ICF) before enrolment. After enrolment and start of the BlackBerry test period for selected subjects, three visits to the clinic were planned: Visit 1 (Baseline), Visit 2 (1 year assessments), and Visit 3 (2 year assessments). In between those visits, about every 3 months, short check-ups by the study nurse were performed on all participants at the study site to check the data collected on paper diaries and to re-issue new diaries and note-books, (see study flowchart: Figure 2).
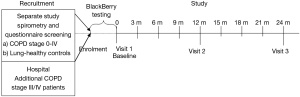
Participants in Sub-group X reported answers on the symptom questionnaire daily (EXACT-PRO) via a BlackBerry PDA device during the whole observation period (12-16). Upon an alert indicating a change in daily symptom pattern the patients were contacted by the clinic, which after obtaining additional information, decided whether the patient had experienced an exacerbation and should visit the clinic within 24-48 hours.
Scheduling extra visits by sub-group X
The clinic centre reviewed each subject’s daily symptoms (provided by BlackBerry PDA) and tracked the symptoms when clearly deviant from the preceding daily pattern by a telephone call. When respiratory symptoms met one of the following pre-determined criteria, an Extra Clinic Visit was scheduled within the following 24 to 48 hours:
- Two days of symptom worsening;
- The subject had to see a doctor or a nurse for breathing problems;
- The subject reported they had symptoms of a cold or flu.
For an extensive description on the decision criteria for an extra visit we refer to Appendix A.
Study assessments
At visits 1, 2 and 3 in all subjects the following assessments were performed: Symptom questionnaires, Medical history, vital signs, Chest X-ray, blood collection for haematology, biochemistry and biomarker analysis and lung function tests (see also Table 2) (17-24). Subjects in Subgroup X were requested to pay an extra visit to the clinic in the case that they fulfilled the pre-determined criteria for a call by the study nurse and the answers to the control questions by the nurse that indicated that an exacerbation had started (see above and Appendix A). At these extra visits nasal and throat swabs, induced or spontaneous sputum and blood were collected as outlined in Table 1. For Sub-group M at visits 2 and 3 and at an exacerbation measure of muscle strength and a Dexa scan were performed (see Table 1) (25).
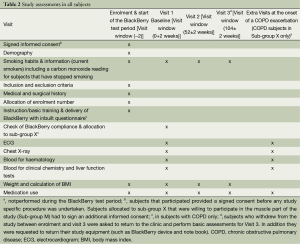
Full table
In addition to these subject oriented assessments environmental data were collected: i.e., daily levels of nitrogen oxide (NO), carbon monoxide (CO), sulphur dioxide (SO2), particulate matter (PM10), air humidity and air temperature.
For genetic analysis of exacerbations subjects participating in sub-group X were asked to sign an additional ICF and blood samples for this particular purpose were stored at −70 °C until analysis.
Data collection and management
eCRFs were entered in a central database and a copy of the eCRF was archived at the study site. Coding had been according to agreed coding conventions (MedDRA). After review, editing and Source Data Verification (SDV), the data were then locked to prevent further editing. After clean file, all data were extracted into SAS data sets for storage and statistical analysis. Data verification and consistency checks were performed on the locked data to ensure data quality by the data management centre.
Data associated with biological samples were collected separately and at a later stage merged with the final dataset.
Genotype data generated in this study were stored in the AstraZeneca genotyping LIMS database, separate from the database used for the main study.
Sample size
Since this study is not interventional and no treatment has been administered, no proper power calculations based on a particular hypothesis have been performed. The study was rather sized to be able to estimate various quantities with a sufficient precision. An adequate sample size for the study was calculated based on data from other internal AZ studies that investigated the causative factors for COPD exacerbations in Canada and used published results based on simulations of the generalized linear mixed effect models with Poisson outcome in order to estimate the effect sizes of fixed effects. The sample size calculation has also taken into consideration the minimum number of subjects that is required to ensure sufficient numbers of sub-group X subjects for further subgroup analyses. These sample size calculations indicated that when for sub-group X, n=50 per GOLD stage group and n=50 for age-matched controls were chosen, the study would have sufficient sample size.
Statistical analysis
For testing of group differences Mann-Whitney tests will be used for all continuous variables and Fisher’s exact tests will be used for categorical variables. Since this is an exploratory study no correction for multiple testing will be performed. Due to smaller numbers in the various subgroups statistical analysis in the whole study population group is expected to have a greater power than similar analysis performed in sub-groups X or M.
Results
Patient demographics at study start
Basic characteristics for the total population, sub-group X, sub-group M, different GOLD severity stages and healthy control subjects are depicted in Table 3. Sub-group X showed similar demographic characteristics to the total population. At study start all patient groups consisted of more than 70% men whereas the control groups consisted of more than 60% women. When patients were subdivided according to GOLD stage (Table 4) it appeared that subjects with COPD stage 1-4 had an increased number of pack years compared to the controls. As expected the proportion of current smokers and ex-smokers was larger in the COPD groups than in the control groups. The proportion of never smokers in GOLD stage 3/4 seemed in general to be much lower than in the other groups. In all groups the proportion of women that were never smokers was higher than men in lung-healthy subjects and those with COPD at risk (GOLD stage 0). Many of the lung function parameters were changed in the COPD patient groups in line with their GOLD stage (Table 5).
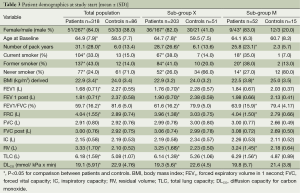
Full table
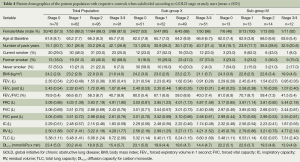
Full table
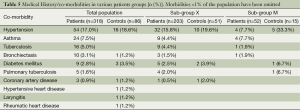
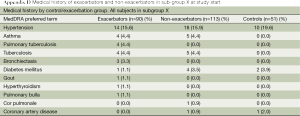
Table 5 depicts the co-morbidities in subjects with COPD stage 0-4 vs. lung-healthy controls.
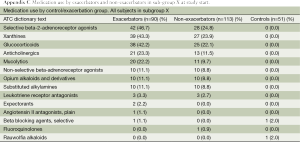
Medication use at study start is depicted in Table 6.
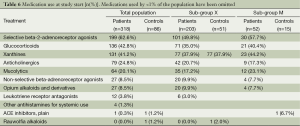
Enrolment and completion
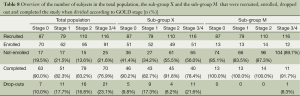
For the total population, sub-group X and sub-group M the number of recruited and enrolled patients are depicted in Table 7. The percentage of patients in the total population and sub-group X that after enrolment successfully completed the study was ≥78%. In sub-group M the percentage of patients that completed the study was ≥98%, indicating that most dropouts took place in the first year of the study.

Full table
When the patients in the total population, sub-group X and sub-group M were further subdivided according to GOLD stage it appeared that most dropouts were registered in GOLD stage 3 and 4 and the least dropouts were registered in GOLD stage 0 (see Table 8). The difference was around 10%.
Full table
Exacerbators and non-exacerbators in sub-group X
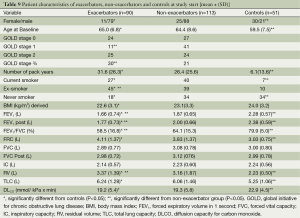
After completion of the study the COPD patients selected for sub-group X could be separated into a group of exacerbators and a group of non-exacerbators over the 2-year observation period.
Their characteristics are shown in Table 9 and Appendix C-D. There were significantly more exacerbators than non-exacerbators in the GOLD stage 3 and 4 group by than in the GOLD stage 1 group. In general lung function outcomes were lower in the exacerbators than in the non-exacerbators and controls.
Full table
Exacerbation frequency in the observation period
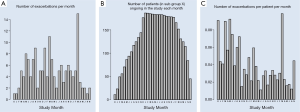
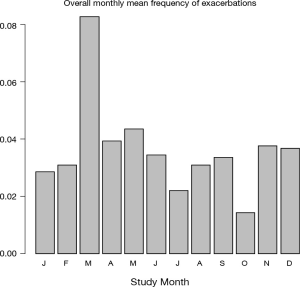
The occurrence of exacerbations, based on extra visits, in the observation period expressed as the absolute total number of exacerbations per month, split into exacerbation numbers, the number of patients that were in the study each month and the mean number of exacerbations per patient calculated per month are graphically presented in Figure 3. Since the graphs seemed to indicate that in certain months of the year the exacerbations were more frequent than in others the mean exacerbation frequency per month was calculated. Figure 4 depicts that over the total observation period the mean number of exacerbations per month was on average highest in the month of March and lowest in the month October.
Discussion
The study design described herein was carried out in Guangzhou, a large industrial city in China. The area is heavy industrialized and densely populated and therefore an excellent area to study the impact of air pollution, viral infections and bacterial infections as trigger factors of COPD exacerbations. The study design primarily focussed on obtaining a better insight in the occurrence of exacerbations and the different trigger factors that cause them. Since the study monitored the patients very closely for 2 years it will allow the analysis of seasonal influences on the occurrence of exacerbations. Daily measures of air pollution, temperature and humidity will allow establishing an association between these factors and an exacerbation as well. Due to the collection of nasal, throat and sputum samples at the planned and extra visits at an exacerbation, we may determine by RNA analysis what microbiological species are present in patients experiencing an exacerbation. Moreover, determination of a range of biomarkers may reveal new markers that are associated with an exacerbation. The study design described herein not only encompassed the occurrence and trigger factors of exacerbations but also aimed to monitor COPD worsening in itself, not only as a consequence of the exacerbations. For this purpose, CT imaging and QoL measures were incorporated and performed. The longitudinal monitoring of exacerbations of the COPD patients over a period of 2 years may also allow describing different COPD exacerbation phenotypes as well as different exacerbation patterns. Since exacerbations could impact skeletal muscle function the study incorporated a small group of COPD patients where muscle and muscle function changes in relation to an exacerbation were tested over a 1 year period in the second year of the study.
In this sense the study design and its complete outline incorporating all these variables may be termed rather unique for China.
To be able to exactly determine the time point of the occurrence of an exacerbation over the observation period a daily symptom scoring system was incorporated. The use of a BlackBerry PDA device to monitor daily symptoms based on an extensive questionnaire has allowed the identification of an alert for an exacerbation rather at an early stage. By contacting the patient and asking for additional information a proper judgment of the status of an exacerbation was made and on the basis of this information a balanced decision was made on whether an extra visit to the clinic was required. This decision making tool was shown to improve the determination of the onset, occurrence and severity of a COPD exacerbation (13-15).
Taken together, this study focused on many aspects related to exacerbations in COPD. In this respect this study differed from the ECLIPSE study where such a study focus was not present at study start (17).
Due to the fact that it was a large single centre study, all assessments that were performed were fully standardised and created the least possible variability in outcomes. In all subjects a fixed number of assessments were performed at yearly intervals distributed over the 2 year observation period, giving a proper impression about disease stability and progression.
In addition to the study design the patient demographics at study start indicated the focus of the study design. All planned groups were recruited to a satisfactory number and the final distribution in all groups was well-balanced as described in the results section. Careful analysis of the demographics of the various groups revealed some findings that may have to be taken into account in further analysis of the data. The first finding was that in the at risk patient group and in the control group the ratio female/male was strongly in favour of females whereas in the more severe COPD patient groups it was completely the reverse. In the latter groups there was strong male preponderance. There is evidence in the literature that the progression of COPD is different in males and females and therefore the observed gender difference in the various groups will have to be taken into account as a confounding factor in forthcoming analyses.
When analysing the smoking habits of the various groups with regard to the within group distribution of current smokers, ex-smokers and never smokers, an imbalance in the distribution between the groups was observed. In GOLD stage 3/4 patients and to a lesser extent GOLD stage 2 COPD patients there existed a considerable proportion of current smokers and ex-smokers and almost no never smokers whereas in the at risk groups and in the controls it was completely the reverse. When connecting gender with smoking habits it appeared that almost all never smokers were females whereas hardly any males fell within this category.
Study completion percentages for the total patient population and Sub-group X were ≥78% and the dropout rates were slightly higher for GOLD stage 3 and 4 than for GOLD stage 0. The reported dropout rates will however not have a critical impact on further statistical analysis of the results.
After completion of the study it appeared that in sub-group X there were n=90 exacerbators and n=113 non-exacerbators. The exacerbators were on average more severe COPD patients than the non-exacerbators as was reflected by their GOLD stage and their lung function parameters at study start. This exacerbator group will be analyzed in forthcoming studies more extensively.
Exacerbations were reported to occur throughout the whole observation period of 2 years. On average the highest number of exacerbations was recorded in March and the lowest number of exacerbations was recorded in October.
Full analysis of the data is currently in progress. Expected outcomes of this study are:
- Insight into the frequency of exacerbations in the exacerbator COPD patient group;
- Insight into the most important trigger factors of those exacerbations with the question in mind: are viral triggers more important than environmental factors;
- Identification of biomarkers that change during an exacerbation and may be associated with an exacerbation;
- Identification of biomarkers for disease progression with the help of CT data and soluble biomarkers;
- Identify markers for disease progression: is it dependent on smoking or passive smoking habits and/or the occurrence of exacerbations;
- Identification of patient phenotypes: do frequent exacerbators have a different genetic background to less frequent exacerbators and non-exacerbators;
- Identification of exacerbation phenotypes: can different exacerbation patterns be distinguished;
- Evaluation of the accuracy of the EXACT-PRO tool in predicting an exacerbation when used properly by a Chinese population over an observation period of 2 years;
- Can advanced CT imaging analysis assist in characterising COPD patients that may be at risk of developing an exacerbation;
- These are just some of the potential topics we plan to address in forthcoming papers using the here described study results and the obtained patient dataset.
Acknowledgements
Competing interests: PLBB, CM, MvG, AN, SH, MH, ZT, UN and YG are all employed by AstraZeneca, YZ, JZ, RZ, JX, PR, RC, NZ are employed by the Chinese Government.
Funding: This project was supported by an unrestricted grant from AstraZeneca.
Author’s contributions: PLBB and YZ drafted the manuscript and contributed equally in writing and interpretation of the results. YZ, RZ, JZ, JX, PR, RC and NZ recruited the patients and collected the physiological data and patient samples. All authors contributed to the analysis of the data and preparation of the final manuscript. YZ, UN, YG, RC, JZ and NZ conceived the design and idea of the study and NZ is the principal investigator who takes responsibility for the integrity of the work as a whole, from inception to published article
Disclosure: The authors are indebted to the following individuals: for study monitoring and management: Eva Hägglund, Susanna Johansson. Jonathan Lewis, Paul Newbold, Suzanne Tan and Briony Wilkinson. For statistics and programming: Paul Dodson, Andrew Lloyd, Gunnar Magnusson and Meizhuo Zhang. For imaging: Lars Nordenmark, For translational biomarkers: Margaret McCormick, Paul Rugman, Magdalena Rhedin, Jin Qian, Renhong Tang, Christina Wang, Jenny Xia, Li Zheng and Changting Liu. The authors declare no conflict of interest.
Appendix A
Symptom Monitoring with the BlackBerry Device and Trigger for Suspected Onset of an Exacerbation (including the Exacerbations of Chronic Obstructive Pulmonary Disease—Patient Reported Outcome (EXACT-PRO) with the Additional Questions used on the BlackBerry Device)
Symptom monitoring
Visit 1
At the Enrolment Visit (Visit 1), consenting COPD subjects will be given further information about the study and they will provide daily respiratory symptom data through an Internet secure wireless Personal Digital Assistant (PDA)—the BlackBerry. They will receive instructions in the use of the BlackBerry PDA daily diary system. They will be asked to complete the EXACT questionnaire and 4 additional questions (see Section 3) on the BlackBerry on a daily basis over the next 2-week test period. Data entry is entirely through the navigation scroll wheel and no keyboard data entry is required. These data will enable understanding of the dynamics of respiratory symptoms and their relation to signal events involving social gatherings and possible high-risk periods for transmission of respiratory infections.
Visit 2 to visit 4
At Visit 2 subjects assessed as being able to use the BlackBerry adequately will be allocated into Sub-group X of the study and will continue to enter daily respiratory symptoms via a secure Internet connection using the BlackBerry PDA throughout the study period. The daily diary questionnaire will not have any identifiable information on it except the subject enrolment number and the diary date. Data transmission will be encrypted and fully secure.
Subjects will complete the daily diary every day for 24 months, starting at the time of enrolment into the study. Data will be reviewed electronically by the centre on a daily basis, via a secure web site showing summarised data in both tabular and graphical formats. Flags will be set in the host database receiving the daily diary data to warn study nurses or the principal or co-investigators that possible respiratory symptom exacerbation events are occurring or if medical intervention has been obtained for breathing problems. Subjects will be contacted whenever diary information is missing for 2 days in a row and given the support or backup needed to get back on track.
Subjects whose daily symptom diaries suggest that a respiratory virus infection (RVI) or respiratory symptom exacerbation has occurred will be interviewed to determine possible opportunities at which they may have been infected or exposed to other agents.
Trigger for respiratory symptom exacerbation
If the subject experiences a worsening in symptoms that meet pre-defined criteria they will be telephoned by the study nurse or investigator for further questioning to confirm onset of an exacerbation (see Section 2.1). On positive response to questioning the study nurse or investigator will arrange an Extra Clinic Visit within the following 24 to 48 hours.
Defining criteria for suspected onset of an exacerbation and confirmation of an exacerbation
On receiving the diary card data, the study site staff will review each subject’s daily symptoms and track these symptoms over the course of time.
When respiratory symptoms meeting pre-determined criteria occur subjects will be contacted by telephone by the study site staff as soon as possible to confirm onset of an exacerbation. The predetermined criteria, obtained from the daily diary data to trigger a telephone call from the study nurse/investigator to the subject will be one of the following:
Symptom score change according to algorithm
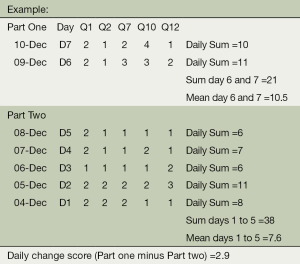
Questions 1, 2, 7, 10 and 12 from the BlackBerry diaries (see Section 3 for questions), will be used to determine the likely onset of an exacerbation. Changes in the symptom scores for these questions according to the algorithm below, will automatically flag (in the web-based monitoring system) the need to contact a subject.
((Day 6 sum + Day 7 sum)/2) - (Average of daily sums for day 1,2,3,4,5 / Number of Days) = X
The flag will be raised on each day that X >2
Note: The calculation will run as long as data for days 1 or 2 is present, whereas data for at least three days is required for days 1 through 5.
Legend: Not at all =1, Slightly =2, Moderately =3, Severely =4, Extremely =5 and Too breathless to do these =6.
The maximum daily score for questions 1,2,7,10 and 12 is 26.
The subject saw a doctor or a nurse for breathing problems
The subject reports they have symptoms of a cold or flu
In addition to the symptom score changes according to the algorithm, the system will also raise a flag to indicate a telephone call may be necessary if the response to Questions 16 and 17 is “Yes”, i.e., the subject saw a doctor or a nurse for breathing problems or the subject reports they have symptoms of a cold or flu. Such a response could be indicative of the start of an exacerbation so may require a telephone call to establish if this is the case, even if the symptom scores have not increased. If however the subject is responding “Yes” to these questions repeatedly and it has been established on a previous day that they are not experiencing an exacerbation and their symptom scores are not rising then a telephone call may not be necessary.
At the telephone contact, the study nurse/investigator will ask subjects:
- If they think their breathing is worse (increased dyspnoea) beyond their normal day-to-day variation and if they have an increase in sputum volume (+ sputum purulence);
- To confirm that medical attention was sought for “breathing problems”;
- To confirm that subjects have respiratory tract infections.
If the answer is “yes” to any of the above then an Extra Clinic Visit will be scheduled within the following 24 to 48 hours. At the Extra Clinic Visit, exacerbation questions and assessments will be completed.
Questions for daily diary reporting
The BlackBerry device will be used to report and record a series of 18 questions on a daily basis. The first 14 questions will be taken from the Exacerbations of chronic obstructive pulmonary disease - patient reported outcome (EXACT-PRO) questionnaire. This is a questionnaire that is being developed across consortia of industry and the FDA for detecting onset and frequency of exacerbations. The remaining 4 questions will ask the subject about the colour of their phlegm, whether they have symptoms of an upper respiratory tract infection, whether they have recently seen a doctor for breathing problems and if they need to be contacted by the study site staff for any reason.
The 18 questions on the BlackBerry device are as follows:
1. Did your chest feel congested today?
- Not at all
- Slightly
- Moderately
- Severely
- Extremely
2. How often did you cough today?
- Not at all
- Rarely
- Occasionally
- Frequently
- Almost constantly
3. How much mucus (phlegm) did you bring up when coughing today?
- None at all
- A little
- Some
- A great deal
- A very great deal
4. How difficult was it to bring up mucus (phlegm) today?
- Not at all
- Slightly
- Moderately
- Quite a bit
- Extremely
5. Did you have chest discomfort today?
- Not at all
- Slight
- Moderate
- Severe
- Extreme
6. Did your chest feel tight today?
- Not at all
- Slightly
- Moderately
- Severely
- Extremely
7. Were you breathless today?
- Not at all
- Slightly
- Moderately
- Severely
- Extremely
8. Describe how breathless you were today:
- Unaware of breathlessness
- Breathless during strenuous activity
- Breathless during light activity
- Breathless when washing or dressing
- Present when resting
9. Were you short of breath today when performing your usual personal care activities like washing or dressing?
- Not at all
- Slightly
- Moderately
- Severely
- Extremely
- Too breathless to do these
10. Were you short of breath today when performing your usual indoor activities like cleaning or household work?
- Not at all
- Slightly
- Moderately
- Severely
- Extremely
- Too breathless to do these
11. Were you short of breath today when performing your usual activities outside the home such as gardening or errands?
- Not at all
- Slightly
- Moderately
- Severely
- Extremely
- Too breathless to do these
12. Were you tired or weak today?
- Not at all
- Slightly
- Moderately
- Severely
- Extremely
13. Last night, was your sleep disturbed?
- Not at all
- Slightly
- Moderately
- Severely
- Extremely
14. How scared or worried were you about your lung problems today?
- Not at all
- Slightly
- Moderately
- Severely
- Extremely
15. What colour was your phlegm today?
- Did not cough up phlegm
- Clear or white or milky
- Deep yellow or green
16. Have you had any of the symptoms of a cold or flu shown below today?
- No, have not had any cold or flu symptoms
- Yes, have a sore throat, fever, shivers or chest congestion
17. Did you see a Doctor or Nurse today for breathing problems or a cold?
- No
- Yes
18. Is there anything about the diary that you’d like the study team to contact you about?
- No
- Yes
Appendix B
Recruitment
It is anticipated that the majority of subjects with COPD (including those “at risk” for COPD) and subjects without any chronic respiratory symptom or chronic respiratory disease (lung-healthy controls) will be recruited from a population previously identified by spirometry and questionnaire screening in a separate study conducted by the Guangzhou Institute of Respiratory Diseases. There may be a need to complement the study population with known patients with COPD from the Guangzhou Institute of Respiratory Diseases, particularly patients with severe COPD (GOLD stage III-IV).
Eligibility criteria
Inclusion criteria
For inclusion in the study subjects must fulfil all of the following criteria:
- Subjects with COPD or “at risk” of COPD;
- Provision of informed consent prior to any study-specific procedure;
- Resident of Guangzhou area;
- Men and women aged 40-79 years;
- Either
- A diagnosis of COPD at stage I to IV according to GOLD 2008 (Table 1) or being “at risk” for COPD (i.e., GOLD stage 0) according to the following definition;
- Chronic cough and sputum production for at least 3 months in each of two consecutive years without any other condition explaining the cough and a post-bronchodilator FEV1/FVC ≥0.7 and FEV1 ≥80% PN;
- Able to read and fluent in local language, in order that they are able to complete the study questionnaires.
Control subjects
- Provision of informed consent prior to any study-specific procedure;
- Resident of Guangzhou area;
- A post-bronchodilator value of FEV1/FVC ≥0.7 and FEV1 ≥80% PN and without any respiratory disease or chronic respiratory symptoms;
- Men and women aged 40-79 years, age and gender matched with the COPD or “at risk” of COPD study population;
- Able to read and fluent in local language, in order that they are able to complete the study questionnaires.
Exclusion criteria
Subjects must not enter the study if any of the following exclusion criteria are fulfilled:
- Subjects with COPD or “at risk” of COPD
- Asthma (e.g., as defined by GINA 2008) in the absence of COPD symptoms or diagnosis;
- Significant disease or disorder, e.g., cardiovascular, pulmonary other than COPD, gastrointestinal, liver, renal, neurological, musculoskeletal, endocrine, metabolic, malignant, psychiatric, major physical impairment which, in the opinion of the investigator, may either put the subject at risk because of participation in the study, or influence the results of the study, or the subject’s ability to participate in the study;
- Chronic respiratory failure including requirement for regular oxygen therapy;
- A severe exacerbation of COPD (defined as use of antibiotics and/or oral/systemic glucocorticosteroids or hospitalisation related to COPD worsening) within 1 month prior to Visit 1;
- Participation in another study involving drug administration or blood donation (>500 mL) within 3 months of Visit 1;
- Planned hospitalisation during the course of the study;
- Involvement in the planning and conduct of the study (applies to both AstraZeneca staff or staff at the study site);
- Change of maintenance medication or start of new medication for COPD within 1 month prior to Visit 1.
Control subjects
- Significant disease or disorder, e.g., cardiovascular, pulmonary, gastrointestinal, liver, renal, neurological, musculoskeletal, endocrine, metabolic, malignant, psychiatric, major physical impairment which, in the opinion of the investigator, may either put the subject at risk because of participation in the study, or influence the results of the study, or the subject’s ability to participate in the study;
- Any chronic respiratory symptom or chronic respiratory disease;
- Participation in another study involving drug administration or blood donation (>500 mL) within 3 months of Visit 1;
- Planned hospitalisation during the course of the study;
- Involvement in the planning and conduct of the study (applies to both AstraZeneca staff or staff at the study site).
Exclusion criteria for sub-group M
Subjects must not be entered into Sub-group M if any of the following exclusion criteria are fulfilled:
- Regular treatment with any anti-platelet or anti-coagulant medication, e.g., heparin, warfarin or aspirin
- Any impairment that may prevent them from attending the hospital for the muscle biopsy assessment.
Restrictions during the study
Any subject allocated to Sub-group M who is taking non-steroidal anti-inflammatory drugs (NSAIDs) should have this medication stopped 2 weeks prior to the muscle biopsy assessment.
Any subject in Sub-group M who commences on anti-platelet or anti-coagulant medication, e.g., heparin, warfarin or aspirin during the second year of the study should not have the muscle biopsy performed.
Discontinuation of subjects
Subjects who withdraw from the study between Visits 1 and 4 should be asked to return to the clinic to perform basic assessments aimed for Visit 4 and also return their study equipment (such as the BlackBerry device and paper diary/note-book).
Criteria for discontinuation
Subjects may be discontinued from the study at any time, at the discretion of the investigator. Subjects are free to discontinue their participation in the study at any time.
If a subject discontinues participation in the study, then his/her enrolment number cannot be issued to another subject.
Subjects who withdraw consent to the use of their biological samples will be withdrawn from the study if they are in Sub-group X or Sub-group M.
Full table
Full table
References
- Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256-76. [PubMed]
- Pauwels R, Calverley P, Buist AS, et al. COPD exacerbations: the importance of a standard definition. Respir Med 2004;98:99-107. [PubMed]
- Seemungal TA, Donaldson GC, Bhowmik A, et al. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;161:1608-13. [PubMed]
- Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106:196-204. [PubMed]
- Mallia P, Message SD, Kebadze T, et al. An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations: a pilot study. Respir Res 2006;7:116. [PubMed]
- Thomsen JL, Parner ET. Methods for analysing recurrent events in health care data. Examples from admissions in Ebeltoft Health Promotion Project. Fam Pract 2006;23:407-13. [PubMed]
- Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med 2007;176:753-60. [PubMed]
- Zhou Y, Wang C, Yao W, et al. COPD in Chinese nonsmokers. Eur Respir J 2009;33:509-18. [PubMed]
- Yin P, Jiang CQ, Cheng KK, et al. Passive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort Study. Lancet 2007;370:751-7. [PubMed]
- Liu S, Zhou Y, Wang X, et al. Biomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural South China. Thorax 2007;62:889-97. [PubMed]
- Ko FW, Woo J, Tam W, et al. Prevalence and risk factors of airflow obstruction in an elderly Chinese population. Eur Respir J 2008;32:1472-8. [PubMed]
- Stone AA, Shiffman S, Schwartz JE, et al. Patient compliance with paper and electronic diaries. Control Clin Trials 2003;24:182-99. [PubMed]
- Wedzicha JA, Agusti A, Vestbo J, et al. Novel detection of exacerbations of COPD with patient reported outcome (EXACT-PRO) and Blackberry (BB). European Respiratory Societ nbnny Annual Congress, September 18-22, 2010, Barcelona. Abstract #395.
- Halpin DM, Laing-Morton T, Spedding S, et al. A randomised controlled trial of the effect of automated interactive calling combined with a health risk forecast on frequency and severity of exacerbations of COPD assessed clinically and using EXACT PRO. Prim Care Respir J 2011;20:324-31, 2 p following 331.
- Mackay AJ, Donaldson GC, Patel AR, et al. Detection and severity grading of COPD exacerbations using the exacerbations of chronic pulmonary disease tool (EXACT). Eur Respir J 2014;43:735-44. [PubMed]
- Budd C. Using a Blackberry to support clinical practice. Comput Inform Nurs 2007;25:263-5. [PubMed]
- Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). Eur Respir J 2008;31:869-73. [PubMed]
- ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [PubMed]
- Borg, G. The Borg CR10 Scale Folder. A method for measuring intensity of experience. Hasselby, Sweden. Borg Perception, 2004.
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005-12. [PubMed]
- Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med 1991;85 Suppl B:25-31; discussion 33-7.
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988;93:580-6. [PubMed]
- Steiner MC, Barton RL, Singh SJ, et al. Bedside methods versus dual energy X-ray absorptiometry for body composition measurement in COPD. Eur Respir J 2002;19:626-31. [PubMed]
- Barreiro E, Schols AM, Polkey MI, et al. Cytokine profile in quadriceps muscles of patients with severe COPD. Thorax 2008;63:100-7. [PubMed]