Meta-analysis of the diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules
Introduction
Lung cancer is maintaining as the leading cause of cancer mortality worldwide (1). A low cure rate of lung cancer is not only attributed to intrinsic aggressive biological behavior, but also little attention to lung cancer screening. A large number of patients with lung cancers present locally advanced or metastatic disease when diagnosed. Since then, early detection of small lung nodules is urgently needed, and may improve lung cancer mortality (2). And the cure rate for lung cancer in stage I is 70% (3).
Current methods of diagnosing lung cancer are limited by several factors, such as the size, location, and morphology of the suspected lesion, the presence of metastasis, and the clinical history and status of the patient. Although surgical biopsy and resection is the best option for the treatment of malignancy, the probability of malignancy is uncertain, and moreover, some high-risk cases are not suitable for surgery. In this respect, computed tomography (CT) screening programs are widely recommended for a large number of potentially resectable small lung nodules can be identified by CT scanning. However, the high frequency of using CT scanning in cardiopulmonary disease is accompanied with high proportion of false-positive CT findings, reaching to 70% (4), which requires further histological confirmation. In addition, CT screening is advent for a significant number of lung cancer patients, who may be medically inoperable due to comorbid medical illness or poor pulmonary function (5,6).
Diagnosis of peripheral pulmonary lesions (PPL) is more challenging, since PPLs account for 25-30% of all lung cancers and are defined as lesions located beyond the segmental bronchus that are less than 2 cm in diameter (7). Although the flexible bronchoscopy is the least invasive diagnostic tool available to the clinician, the value of this conventional bronchoscopy is limited by the size and location of the lesions of interest. The diagnostic yield of the flexible bronchoscopy for peripheral lung nodules is low, ranging from 14% to 62% (8), highly dependent on the size and location of lung lesions. In these cases, other modalities such as CT-guided biopsy can help determine the malignancy of peripheral lung nodules. However, this modality is generally applicable to lung lesions located in the outer third of the lung, and more severely, led to high occurrence of pneumothorax in patients with advanced chronic obstructive pulmonary disease (9,10). Hence, a guided endoscopic approach may be the best alternative approach for patients with lung nodules. To this end, electromagnetic navigation bronchoscopy (ENB) is a promising option in the diagnosis of lung nodules.
ENB, a newly developed technology, is recognized as an ideal platform to biopsy, localize, and even mark the small peripheral lesions for treatment (11). More importantly, ENB has allowed thoracic surgeons not only to localize these small deep lesions but to biopsy these lesions intra-operatively when combined with rapid on-site cytological evaluation (ROSE) (12). The ENB system consists of four essential components, which are (I) computer software that creates a three-dimensional (3D) and virtual bronchoscopy reconstruction from CT-scan data of patients airways; (II) an electromagnetic location board generating a low-dose magnetic field surrounding the patients’ chest; (III) a sensor probe that located in the electromagnetic field to guide a steerable endoscopic probe to peripheral lung lesions; and (IV) an extended working channel that enables the placement of the bronchoscopic tools to biopsy the lung periphery (5).
This minimally invasive approach for sampling a peripheral lung nodule has not been well confirmed. The diagnostic yields with ENB were inconsistently reported in different research group, After Gex et al. released a meta-analysis on the evaluation of ENB for lung nodules (8), four more manuscripts were released to report the utility of ENB for the diagnosis yield of peripheral lung lesions (7,13-15). Hence, in this study, we updated the meta-analysis to assess the diagnosis accuracy and safety of ENB for the peripheral lung nodules.
Materials and methods
Data sources
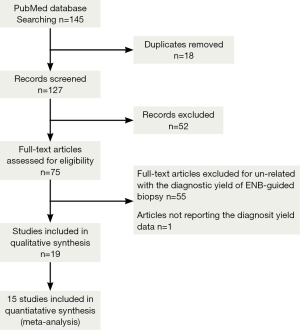
A total of 145 literatures were found after the key words, “electromagnetic navigation bronchoscopy”, “bronchoscopy”, “peripheral nodule” or “lung cancer” were used to search on the PubMed database from 2000 to 2015. The search was limited to human subjects and to English language studies.
Study selection
Review and editorial articles were excluded from the meta-analysis, but the reference lists of these papers were searched manually for additional relevant articles. Studies were included in this meta-analysis when it met the following criteria: (I) each trial enrolling more than ten patients; (II) the diagnostic yield of ENB was reported for peripheral lung nodules or lesions without any restriction; (III) the enrolled patients with peripheral nodules were confirmed by radiographic evidence.
Data extraction and quality assessment
Two investigators independently reviewed the enrolled manuscripts for eligible articles and extracted the data using a standardized form. Disagreements were solved through discussion with the third person to reach a consensus. Further, the quality of included studies was assessed with the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool, consisting of four key domains: patient selection, index test, reference standard, and the flow and timing. The data for each study was entered in the RevMan (Version 5.3, the Cochrane Collaboration), and the methodological quality graph was constructed for all the included studies.
The following data from each study: (I) publication details (title, authors, and other citation details); (II) type of study (prospective or retrospective); (III) exact criteria of inclusion in the study; (IV) type of sedation used and number of biopsies taken; (V) data necessary for diagnostic meta-analysis, such as the number of lesions and the number of diagnoses obtained; (VI) major and minor complications associated with procedure; (VII) reference tests and follow-up duration. The primary outcome was diagnostic yield. ENB success (true positive) was defined as a definitive malignant diagnosis that was yielded by ENB biopsy. When the benign diagnosis was initially yielded by ENB biopsy, which was confirmed by the follow-up treatment, it was also considered as ENB success (true negative). ENB failure represented as an ENB biopsy that yielded a non-diagnostic or a benign diagnosis was overturned when follow-up yielded a malignant diagnosis (false negative).
Statistical analysis
Standard methods recommended for meta-analyses of diagnostic accuracy studies were used (16). All analyses were performed using Meta-DiSc 1.4 (Cochrane Colloquium, Barcelona, Spain) and RevMan (version 5.3). The diagnostic accuracy of ENB was determined by calculating the sensitivity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) for each study. Sensitivity analyses, conducted to check the robustness of the results, were pooled using the fixed effects model, and the PLR, NLR and DOR were pooled using the DerSimonian-Laird random effects model to derive a pooled estimate with 95% confidential index (CI). In addition, a summary receiver operating characteristic curve (SROC) was constructed using the random effects model. Heterogeneity, measuring the extent of inconsistency among the results of the studies, was assessed by the indicator I2. The Cochran Q test was used to find potential heterogeneity factors. All statistical tests were two-sided, and significance was set as P<0.05.
Sensitivity analysis was determined by using the following subgroups, which were considered as potential sources of heterogeneity: (I) prospective vs. retrospective studies; and (II) studies with small or large sample size (small sample defined as studies with less than 25 selected participants); (III) type of sedation; (IV) whether or not the fluoroscopy was used; and (V) whether or not the ROSE was used.
Results
Study selection
A total of 145 studies were yielded after systematic database searches. Following two investigators reviewing, 121 papers were excluded for not meeting the inclusion criteria. Finally, 17 papers on the diagnostic yield and safety of ENB in lung nodules were included in present meta-analysis (7,13-15,17-31). The flow of search strategy is showed in Figure 1.
Study description
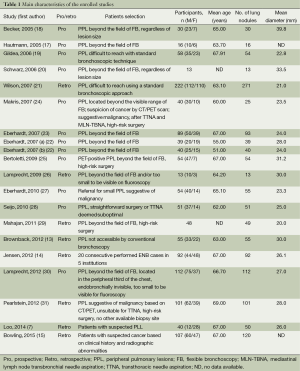
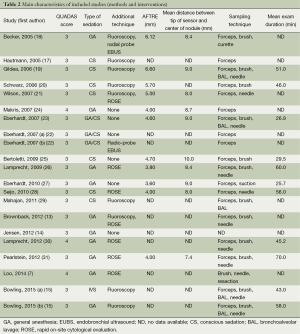
Main demographics of selected patients were depicted in Table 1. A total of 1,161 lung nodules or masses in 1,106 patients were enrolled. The SuperDimension ENB system was used in all studies. Additional techniques or strategies were used in several studies to enhance performance, such as fluoroscopy, endobronchial ultrasound (EBUS) radial probe, ROSE, which were summarized in Table 2.
Full table
Full table
The overall low QUADAS scores indicated that the methodological quality of the enrolled studies was poor (Table 2). Since none of the included studies compared ENB to surgery as a gold standard, QUADAS scores were only evaluated in 6 of the 14 domains. In almost all publications, it was uncertain whether the selected patients were representatives of the patients who were suitable to undergo ENB-sampling, consequently inducing selection bias.
Performance outcomes
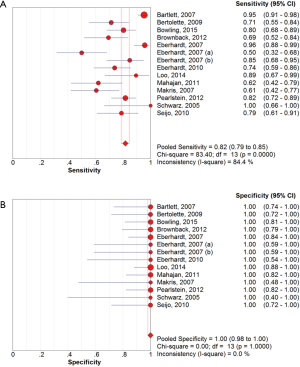
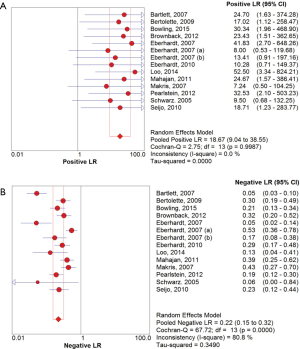
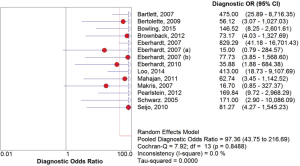
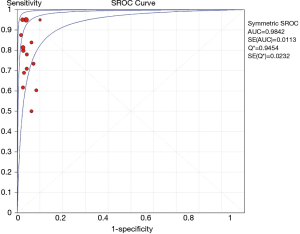
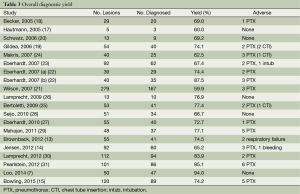
The overall diagnostic yield was summarized in Table 3. Furthermore, 15 out of 19 studies, including 892 patients, reported the TP and TN data of ENB-guided biopsy. Hence, these 15 studies were enrolled for the meta-analysis. The sensitivity of ENB in diagnosis of peripheral lung lesions ranged from 50% to 100%, with the pooled sensitivity being 82% (95% CI, 78-85%). The pooled specificity of ENB procedure was 100% (Figure 2). The pooled PLRs and NLRs were 18.67 (95% CI, 9.04-38.55), and 0.22 (95% CI, 0.15-0.32), respectively (Figure 3). The DOR was 97.36 (95% CI, 43.75-216.69) (Figure 4). The area under the curve (AUC) was 0.98 and the Q-value for the included studies in our meta-analysis was 0.94 (Figure 5), suggesting an overall level of diagnostic accuracy.
Full table
There was clinical heterogeneity caused by the type of study (prospective versus retrospective), varying patients’ inclusion criteria, and the difference in the number of biopsies obtained. Since then, significant statistical heterogeneity for the outcome of sensitivity (I2 =84.4%) and NLRs (I2 =80.8%) were found, while no heterogeneity was detected in other outcomes including specificity, PLRs and DOR.
The first sensitivity analysis was conducted by including only prospective studies (9 studies with 551 patients), which did not explain the heterogeneity (pooled sensitivity, 84%; I2 =88.9%). The next analysis excluded studies less than 20 enrolled patients (13 studies, 866 patients). However, there was no improvement in either the sensitivity (81%) or the heterogeneity (I2 =84.9%). The third analysis included only studies with general anesthesia (10 studies with 553 patients), but there was no influence on the heterogeneity (I2 =80%). The fourth analysis included studies with fluoroscopy as additional technique (5 studies with 373 patients) showed no impact on the heterogeneity as well (pooled sensitivity, 86%; I2 =86%). Final analysis included studies with ROSE as additional technique (6 studies with 439 patients), but there was no decline in heterogeneity (pooled sensitivity, 87%; I2 =83.5%).
Variables influencing the performance of ENB
Like the previous meta-analysis, we systematically reviewed all 19 original studies and identified 16 variables for their postulated influence on ENB’s diagnostic yield (Table 4). Six statistically significant variables were identified by univariate analyses. Two trials reported that location of lower lobe was correlated with decreased yields (15,22), while greater nodule size (15,28), nodule visualization with radial-probe EBUS (22,27), presence of bronchus sign (28), lower registration error (AFTRE) (24), and catheter suction technique (27) were associated with increased yields.
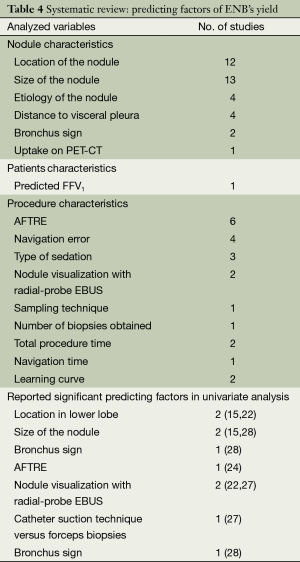
Full table
Safety
Additional complications caused by ENB sampling of peripheral lung nodules were listed in Table 3. A total of 40 pneumothoraces occurred in 681 procedures, in which two cases were induced using transbronchial biopsy, otherwise, none pneumothorax was ENB procedure-related. In addition, minor or moderate bleeding was reported in seven cases, and two of post-procedure respiratory failure were recorded, none of them requiring specific treatment. Other adverse events included two of chest drainage, five of chest pain, three of fever, seven of sore throat, four of hemoptysis, and four of emesis, attributed to sedation or biopsy procedure.
Discussion
The results of this meta-analysis (19 studies, 1,106 patients with peripheral lung nodules) indicated that electromagnetic-guided bronchoscopy has good sensitivity (82%) and excellent specificity (100%) in diagnosing PPLs, suggesting a hugely potential diagnostic value. Given that the likelihood ratios are accepted more useful clinically (32), we calculated the PLR and NLR of ENB-guided diagnosis in PPL patients. The pooled PLR was 18.75, which meant that NPC patients have more than eighteen-fold higher chance of carrying positive ENB-guided biopsies than do healthy people. Since PLR is high above 10, the ENB-guided diagnosing is strong enough to confirm the peripheral lung nodules. Meanwhile, the pooled NLR was 0.22, suggesting that approximately 22% chance of negative ENB-guided diagnostic results is likely to be a false-negative, which is not low enough to rule out peripheral lung lesions. DOR is more widely used to evaluate the test performance as it stands for the single indicator of diagnostic test accuracy, which combines the sensitivity and specificity data into a single number (33). Therefore, a higher value of DOR indicates a better discriminatory test performance. In this meta-analysis, DOR value was 97.36. Furthermore, our AUC was higher to 0.95, all of which suggested an overall high diagnostic accuracy by ENB-guided diagnosis in peripheral lung lesions.
Given that conventional transbronchial needle aspiration (TBNA) lacks a real-time visualization, making it difficulty guiding to the target location, EBUS-driven biopsy has been widely used to increase the diagnostic yield for peripheral lung lesions (34). Besides this, ROSE of transbronchial aspirates has been reported to be capable of providing accurate evaluation, although inadequate specimens were collected, which is another factor limiting the yield of bronchoscopy (35). Based on these, ENB in combination with EBUS or ROSE seems the best alternative diagnosing strategy. Six studies included in this meta-analysis conducted ROSE as additional technique to ENB. The sensitivity and specificity reported in these studies ranged from 85% to 92%, and 96.5% to 100%, respectively. Interestingly, the overall diagnostic yield of ENB-ROSE combination exhibited increasing trend, from 59.9% to 94% (7,13,21,26,28,31). The explanation for this increasing may be overcoming of the learning curve and the optimization of the ENB system.
Finally, the limitation in our study is primarily due to the study design. In a diagnostic accuracy meta-analysis, the results of the index test (ENB-guided diagnosing in this study) needs to be verified against a reference standard ideally on the whole included patients or at least in a random sample. In this case, surgery can be recognized as the gold standard, while high-risk patients are unsuitable for resection. Because of the nature of the ENB, it is difficult to undergo experimental and standard tests. Thus, a reference test was performed only in those with negative index test, whereas all positive results on index test were assumed as TPs. In addition, owing to the nature of the disease, assessment of specificity is difficult, as it is rare to obtain the normal pleura in those with PPL. All of these limitations may be the attribution of the high heterogeneity of this meta-analysis.
In conclusion, ENB is an effective and safe procedure in diagnosing peripheral lung lesion. The outstanding strength of using ENB-guided biopsy is low frequency of adverse events, especially regarding the risk of procedure-related pneumothorax. This new, real-time guidance system seems to be a promising strategy for diagnosis of peripheral lung nodules, which would be confirmed by further analysis and more powered prospective studies.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [PubMed]
- Flehinger BJ, Kimmel M, Melamed MR. The effect of surgical treatment on survival from early lung cancer. Implications for screening. Chest 1992;101:1013-8. [PubMed]
- Swensen SJ, Jett JR, Hartman TE, et al. CT screening for lung cancer: five-year prospective experience. Radiology 2005;235:259-65. [PubMed]
- Weiser TS, Hyman K, Yun J, et al. Electromagnetic navigational bronchoscopy: a surgeon's perspective. Ann Thorac Surg 2008;85:S797-801. [PubMed]
- Manser RL, Wright G, Byrnes G, et al. Validity of the Assessment of Quality of Life (AQoL) utility instrument in patients with operable and inoperable lung cancer. Lung Cancer 2006;53:217-29. [PubMed]
- Loo FL, Halligan AM, Port JL, et al. The emerging technique of electromagnetic navigation bronchoscopy-guided fine-needle aspiration of peripheral lung lesions: promising results in 50 lesions. Cancer Cytopathol 2014;122:191-9. [PubMed]
- Gex G, Pralong JA, Combescure C, et al. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration 2014;87:165-76. [PubMed]
- Shulman L, Ost D. Advances in bronchoscopic diagnosis of lung cancer. Curr Opin Pulm Med 2007;13:271-7. [PubMed]
- Gupta S, Krishnamurthy S, Broemeling LD, et al. Small (</=2-cm) subpleural pulmonary lesions: short- versus long-needle-path CT-guided Biopsy--comparison of diagnostic yields and complications. Radiology 2005;234:631-7. [PubMed]
- Zaric B, Stojsic V, Sarcev T, et al. Advanced bronchoscopic techniques in diagnosis and staging of lung cancer. J Thorac Dis 2013;5 Suppl 4:S359-70. [PubMed]
- Port J, Harrison S. Electromagnetic navigational bronchoscopy. Semin Intervent Radiol 2013;30:128-32. [PubMed]
- Brownback KR, Quijano F, Latham HE, et al. Electromagnetic navigational bronchoscopy in the diagnosis of lung lesions. J Bronchology Interv Pulmonol 2012;19:91-7. [PubMed]
- Jensen KW, Hsia DW, Seijo LM, et al. Multicenter experience with electromagnetic navigation bronchoscopy for the diagnosis of pulmonary nodules. J Bronchology Interv Pulmonol 2012;19:195-9. [PubMed]
- Bowling MR, Kohan MW, Walker P, et al. The effect of general anesthesia versus intravenous sedation on diagnostic yield and success in electromagnetic navigation bronchoscopy. J Bronchology Interv Pulmonol 2015;22:5-13. [PubMed]
- Devillé WL, Buntinx F, Bouter LM, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 2002;2:9. [PubMed]
- Hautmann H, Schneider A, Pinkau T, et al. Electromagnetic catheter navigation during bronchoscopy: validation of a novel method by conventional fluoroscopy. Chest 2005;128:382-7. [PubMed]
- Becker HD, Herth F, Ernst A, et al. Bronchoscopic Biopsy of Peripheral Lung Lesions Under Electromagnetic Guidance: A Pilot Study. J Bronchol 2005;12:9-13.
- Gildea TR, Mazzone PJ, Karnak D, et al. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. Am J Respir Crit Care Med 2006;174:982-9. [PubMed]
- Schwarz Y, Greif J, Becker HD, et al. Real-time electromagnetic navigation bronchoscopy to peripheral lung lesions using overlaid CT images: the first human study. Chest 2006;129:988-94. [PubMed]
- Wilson DS, Bartlett RJ. Improved diagnostic yield of bronchoscopy in a community practice: combination of electromagnetic navigation system and rapid on-site evaluation. J Bronchol 2007;14:227-32.
- Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:36-41. [PubMed]
- Eberhardt R, Anantham D, Herth F, et al. Electromagnetic navigation diagnostic bronchoscopy in peripheral lung lesions. Chest 2007;131:1800-5. [PubMed]
- Makris D, Scherpereel A, Leroy S, et al. Electromagnetic navigation diagnostic bronchoscopy for small peripheral lung lesions. Eur Respir J 2007;29:1187-92. [PubMed]
- Bertoletti L, Robert A, Cottier M, et al. Accuracy and feasibility of electromagnetic navigated bronchoscopy under nitrous oxide sedation for pulmonary peripheral opacities: an outpatient study. Respiration 2009;78:293-300. [PubMed]
- Lamprecht B, Porsch P, Pirich C, et al. Electromagnetic navigation bronchoscopy in combination with PET-CT and rapid on-site cytopathologic examination for diagnosis of peripheral lung lesions. Lung 2009;187:55-9. [PubMed]
- Eberhardt R, Morgan RK, Ernst A, et al. Comparison of suction catheter versus forceps biopsy for sampling of solitary pulmonary nodules guided by electromagnetic navigational bronchoscopy. Respiration 2010;79:54-60. [PubMed]
- Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a Bronchus sign on CT imaging: results from a prospective study. Chest 2010;138:1316-21. [PubMed]
- Mahajan AK, Patel S, Hogarth DK, et al. Electromagnetic navigational bronchoscopy: an effective and safe approach to diagnose peripheral lung lesions unreachable by conventional bronchoscopy in high-risk patients. J Bronchology Interv Pulmonol 2011;18:133-7. [PubMed]
- Lamprecht B, Porsch P, Wegleitner B, et al. Electromagnetic navigation bronchoscopy (ENB): Increasing diagnostic yield. Respir Med 2012;106:710-5. [PubMed]
- Pearlstein DP, Quinn CC, Burtis CC, et al. Electromagnetic navigation bronchoscopy performed by thoracic surgeons: one center's early success. Ann Thorac Surg 2012;93:944-9; discussion 949-50. [PubMed]
- Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ 2004;329:168-9. [PubMed]
- Glas AS, Lijmer JG, Prins MH, et al. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003;56:1129-35. [PubMed]
- Paone G, Nicastri E, Lucantoni G, et al. Endobronchial ultrasound-driven biopsy in the diagnosis of peripheral lung lesions. Chest 2005;128:3551-7. [PubMed]
- Diacon AH, Schuurmans MM, Theron J, et al. Utility of rapid on-site evaluation of transbronchial needle aspirates. Respiration 2005;72:182-8. [PubMed]