Initial thrombolysis treatment compared with anticoagulation for acute intermediate-risk pulmonary embolism: a meta-analysis
Introduction
Acute pulmonary embolism (PE) is the third most common cause of cardiovascular disease-related morbidity, following myocardial infarction and stroke, having a morbidity rate of 1-2‰ (1,2). In the United States, acute PE causes 100,000-180,000 deaths, annually, exceeding mortality due to myocardial infarctions (3), and this number is about 370,000 in six European countries, including France, Germany, Italy, Spain, Sweden, and UK (4). High-risk PE, defined as hemodynamic instability or cardiac shock in acute PE patients, is associated with rapid in-hospital mortality (5,6). The latest guidelines (7-9) and meta-analyses (10,11) suggest that thrombolytic therapy is the most effective treatment for acute, high-risk PE patients.
Intermediate-risk PE, characterized by normtension, right ventricular dysfunction (RVD), and/or myocardial injury, is also a major cause of early death (8,9). However, whether thrombolytic therapy should be chosen for patients with intermediate-risk PE is still debated (12-26). Some literature supports the use of heparin treatment in these patients (12-16), whereas some suggests that these patients are better candidates for early thrombolysis therapy (17-24). Despite the favorable effects of thrombolysis on improving right ventricular (RV) function (19,21,22,26) and pulmonary perfusion (12,19), different studies have not agreed on its benefits for preventing clinical deterioration (20,22,25), reducing pulmonary artery pressure (23,26) and improving comprehensive outcomes (20,24), all-cause death, and bleeding risk. The largest trial comparing thrombolysis and heparin treatment demonstrated that fibrinolytic therapy prevented hemodynamic decompensation, but increased the risk of major hemorrhage and stroke (25).
The unknown composite endpoint of death/PE recurrence, contrasted with heparin treatment, and a high bleeding risk associated with thrombolytic treatment of intermediate-risk PE patients has further aggravated the controversy over which methodology is most suitable. In this meta-analysis, we compared the efficacy and safety of thrombolysis and anticoagulation with that of anticoagulation, alone, for treating this patient population; the meta-analysis included seven randomized controlled trials (RCTs) (19-25).
Materials and methods
Selection of participants, interventions, comparisons, and outcomes
In this study, the participants were intermediate-risk PE patients, defined as having hemodynamic stability and RVD and/or myocardial injury. The primary intervention under investigation was thrombolysis plus anticoagulation, compared against placebo plus anticoagulation or anticoagulation alone.
The primary outcomes included early all-cause mortality, hemorrhagic events, clinical deterioration, and PE recurrent in-hospital or within 30 days of randomization. Hemorrhagic events included major and minor bleeding events, with major bleeding defined as meeting at least one of the following criteria: fatal bleeding, hemoglobin level decreases of ≥2 g/dL, transfusions or intervention for hemodynamic deterioration, and intracranial hemorrhage (21). Any other bleeding event was defined as minor bleeding. Clinical deterioration included sustained hypotension or shock, requiring treatment escalation, including intubation or mechanical ventilation, cardiopulmonary resuscitation, emergency surgical embolectomy, or emergency catheter fragmentation (21).
Secondary outcomes include RV function, mean pulmonary artery pressure (mPAP), and recurrent PE within the first 72 hours after randomization and within the 6-month follow-up period. RV function and mPAP were measured by echocardiography. RV end-diastolic dimension (RV EDD) and the ratio of RV EDD to left ventricular end-diastolic diameter (RV/LV EDD) were chosen to reflect RV function.
Search strategy
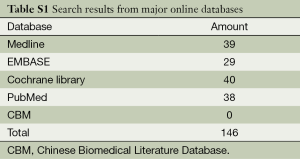
We searched for qualified RCTs among major online databases, including PubMed (1966 through December 2013), EMBASE (1974 through December 2013), MEDLINE (1966 through December 2013), the Cochrane Central Register of Controlled Trials in the Cochrane Library (last issue searched, December 2013), and the Chinese Biomedical Literature Database (CBM, 1978 through December 2013). We used multiple search terms, including “pulmonary embolism”, “thrombolysis” and “anticoagulation”, as well as their root terms in various combinations. We updated the search results before our meta-analysis was finished in April 2014.
Study selection
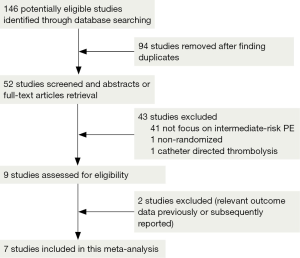
Qualified studies were defined as RCTs comparing thrombolysis plus anticoagulation against placebo plus anticoagulation or anticoagulation, alone, in acute intermediate-risk PE patients. We focused on the efficacy and safety of two treatment strategies via the peripheral veins, but did not evaluate specific drugs or treatment courses. Two authors carefully screened the 52 articles and finally qualified seven studies for inclusion in the meta-analysis.
Assessment of risk of bias of included studies
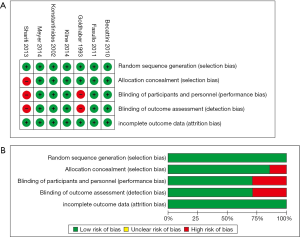
We used Review Manager (version 5.2) software (Cochrane Collaboration, London, UK) to evaluate the risk of bias, including random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessments (detection bias), and incomplete outcome data (attrition bias); the biases were classified as low-, high-, and unclear-risk.
Data extraction and analysis
Two authors independently and carefully reviewed the seven trials and extracted data. Controversial data were discussed, face-to-face to reach agreement. We used Review Manager to perform the meta-analysis. Dichotomous variables were analyzed using odds ratios (ORs) and 95% confident intervals (CIs), and continuous variable analyses involved weighted mean differences (MDs) and 95% CIs. The heterogeneity of the included studies was analyzed with I2 (27); if I2≤40%, homogeneity was accepted and a fixed-effects model was used. If I2>40%, heterogeneity was deduced and a random-effects model or subgroup analysis was used. Funnel plots were introduced to evaluate publication bias.
Results
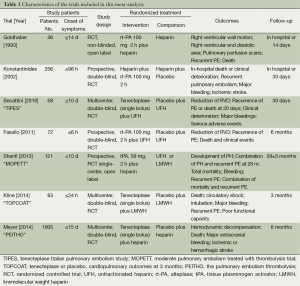
We retrieved 146 potentially eligible studies from PubMed, EMBASE, MEDLINE, the Cochrane library, and CBM Databases. After eliminating duplicates, the full text or abstracts of the remaining 52 studies were screened. By browsing the abstracts or full articles, 45 studies were excluded, as 41 articles were not focus on intermediate-risked, one study was non-randomized, one study was catheter directed thrombolytic and two articles were relevant outcome data previously or subsequently reported. Ultimately, seven studies were included in our meta-analysis. The search results are presented in Table S1 and a flow chart describing the identification of qualified studies is presented in Figure 1. The examined trial characteristics included the publication year, number of study patients, duration of patient symptoms from onset, study design, interventions, comparisons, outcomes and follow-up durations (Table 1). The results of the bias risk analysis, in the included studies, are shown in Figure 2. Figure 2A illustrates the proportion of studies with the judgment for each trial, and Figure 2B presents all of the judgments in a study cross tabulation, by entry.
Full table
Full table
Primary outcomes
Early all—cause mortality
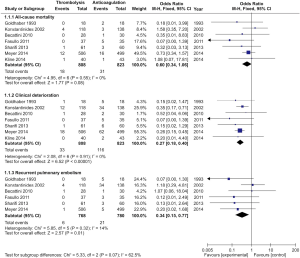
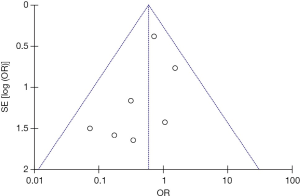
Seven studies were included in the early all-cause mortality (during hospitalization or within 30 days after randomization) analysis. As shown in Figure 3, 1,631 patients with intermediate-risk PE were analyzed, including a thrombolytic group of 808 patients and an anticoagulant group of 823 patients. Early all-cause mortality, after randomization, was lower in the thrombolytic group than in the anticoagulant group (2.2% vs. 3.8%; OR, 0.60; 95% CI, 0.34-1.06), the difference was not statistically different (Z=1.77; P=0.08). All included studies demonstrated good homogeneity (I2=0%) and the meta-analysis was conducted using a fixed-effects model. The funnel plot did not show any obvious publication bias and is presented as Figure 4.
Clinical deterioration events
A total of 1,631 patients from the seven studies were included among those with clinical deterioration events (within 30 days after randomization or during the hospitalization) analysis. As shown in Figure 3, clinical deterioration events were observed in 33 patients in the thrombolysis group and in 116 patients in the anticoagulant group. The rate of clinical deterioration events in the thrombolysis group was, therefore, lower than in the anticoagulant group (4.1% vs. 14.1%; OR, 0.27; 95% CI, 0.18-0.41; P<0.00001). The included studies did not demonstrate heterogeneity (I2=0%), and the fixed-effects model was used for the meta-analysis.
Recurrent PE
Six studies reported recurrent PE within 30 days after randomization or during hospitalization. As shown in Figure 3, 1,548 patients with intermediate-risk PE were analyzed, including 768 patients receiving thrombolytic treatment and 780 patients receiving anticoagulation therapy, only. The recurrent PE rate in the thrombolytic group was significantly lower than that in the anticoagulation group (0.8% vs. 2.7%; OR, 0.34; 95% CI, 0.15-0.77; Z=2.57; P=0.01). All the included studies showed good homogeneity (I2=14%), and the meta-analysis used the fixed-effects model.
Hemorrhagic events
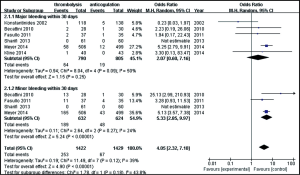
Six studies were included in the hemorrhagic events (within 30 days after randomization or during the hospitalization) analysis. As shown in Figure 5, a total of 1,595 patients with intermediate-risk PE were analyzed, including 790 patients receiving thrombolytic treatment and 805 receiving anticoagulant therapy. As major and minor bleeding has different levels of ascribed importance, in clinical practice, we used a subgroup analysis to evaluate the bleeding events. There were no statistically significant differences in the incidences of major bleeding events between the thrombolytic and anticoagulation groups (8.1% vs. 2.4%; OR, 2.07; 95% CI, 0.60-7.16; P=0.25). The heterogeneity test result was I2=50%, so the meta-analysis used a random-effects model. The incidence of minor bleeding events was higher in thrombolysis group than in the anticoagulant group (29.9% vs.7.7%, OR, 5.33; 95% CI, 2.85-9.97; P<0.00001). The criteria of major bleeding in these six studies were listed respectively in Table S2.

Full table
Secondary outcomes
RV function recovery within the first 3 days
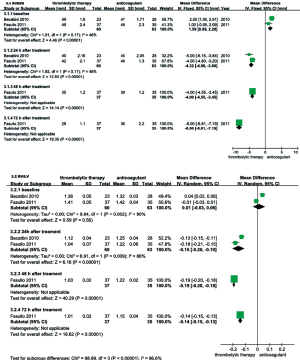
RVEDD, RV/LV EDD, and mPAP were echocardiographically measured to evaluate the RV function of patients with intermediate-risk PE. As shown in Figure 6, the thrombolysis group RVEDD and RV/LV EDD were significantly lower at 24, 48 and 72 hours than observed in the anticoagulant group. As shown in Figure 7, compared with anticoagulant group, the PAP was lower at 24, 48 and 72 hours in the thrombolysis group. These results suggest an improved RV function in the thrombolysis group, within the first 3 days.
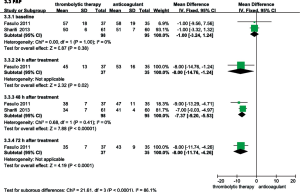
Efficacy in the 6-month follow-up
As shown in Figure 8, only one study compared the thrombolysis and anticoagulant groups relative to recurrent PE during the 6-month follow-up period; no significant differences were observed (OR, 0.15; 95% CI, 0.02-1.28, P=0.08). That study also reported improved RV function in the thrombolytic group. Compared with anticoagulant group, the RV EDD (23±0.2 vs. 26±1.3 mm; MD =−3.00; 95% CI, −3.44 to −2.56; P<0.00001) and the RV/LV EDD (0.67±0.01 vs. 0.88±0.02, MD =−0.21, 95% CI, −0.22 to −0.20; P<0.00001) were significantly lower in the thrombolytic group (22). In addition, two studies reported that the mPAP was lower in the thrombolytic group than in the anticoagulant group (MD, −11.95; 95% CI, −23.71 to −0.19; P=0.05) during the 6-month follow-up period (22,23).

Discussion
From this meta-analysis, we found that, compared with anticoagulation therapy for intermediate-risk PE patients, thrombolysis showed a significant reduction in the clinical deterioration events and recurrent PE, and a lower all-cause mortality rate (P=0.08). The thrombolysis group also showed a survival advantage even though the difference in the mortality rates between the two groups was not statistically significant. The results indicated that RV function and mPAP quickly improved during the first 3 days and were maintained for 6 months in thrombolytic group. Finally, although the total bleeding risk increased with thrombolysis treatment, the incidences of major bleeding events, in the two groups, were not significantly different (P=0.25). These results suggested that intermediate-risk PE patients, without thrombolysis contraindications, may benefit from thrombolytic therapy.
The results of this meta-analysis are in accordance with the findings of previous non-RCT studies, which also supported thrombolytic therapy for intermediate-risk PE patients (28-30). For example, an early study showed that total mortality was significantly reduced in intermediate-risk PE patients receiving thrombolysis. This research reported that the thrombolysis group had a lower incidence of recurrent PE and a higher bleeding risk, but that the risks of fatal and intracranial bleeding were not statistically different from those associated with the anticoagulant group (28). A prospective, non-RCT showed that thrombolytic treatment of this patient population reduced the incidence of clinical complications during hospitalization, and that this protective effect was sustained throughout a 6-month follow-up period. The total mortality was also lower in thrombolysis group than in anticoagulation group (29). Similarly, a retrospective study showed that intermediate-risk PE patients undergoing thrombolytic treatment demonstrated rapid dispend relief, reduced mPAP, and make embolization of recanalization, but that the overall effectiveness, total mortality, and bleeding risk were similar to those associated with anticoagulant therapy (30). Thus, these findings suggest that intermediate-risk PE patients with severe clinical symptoms and a low risk of bleeding should receive thrombolytic therapy.
However, some studies do not support the use of thrombolytic therapy in intermediate-risk PE patients. In 2007, two meta-analyses were published that opposed the use of thrombolysis for submassive PE (15,31). One of them reported that thrombolytic therapy did not reduce mortality in these patients, but increased the costs of treatment and care, and was associated with a potential rise in the risk of bleeding (15). The other compared the health effects and costs of treatment with alteplase (a recombinant tissue-plasminogen activator, rt-PA) and heparin versus heparin alone, for the treatment of intermediate-risk PE patients. The results showed that patients receiving thrombolytic therapy received less benefit and had a higher treatment cost; thus, thrombolysis was not suggested as a first-line therapy (31). However, the numbers of patients enrolled in these meta-analyses were limited and did not benefit from the results of larger and more recent clinical trials.
In 2014, there were three related meta-analyses published. Cao et al. reported that thrombolysis treatment of acute submassive PE reduced neither mortality nor recurrent PE, nor increased bleeding risks (32). The study, however, enrolled only 594 patients and did not include the most recent clinical trial results. Chatterjee et al. reported that the use of thrombolytic therapy for acute PE was associated with lower rates of all-cause mortality and increased the risks of major bleeding and intracranial hemorrhage. In the subgroup analysis, specifically enrolling only the intermediate-risk PE patients, thrombolysis reduced mortality, but increased the incidence of major bleeding (33). Although the mortality rates at different time points were summarized, our meta-analysis supported their results, but our bleeding risk results conflicted with those of Chatterjee et al. (33). In our meta-analysis, the risk of major bleeding was analyzed not including the data in the ULTIMA trail (34) and the Goldhaber et al.’s research (19). In ULTIMA trail, alteplase was administered through a catheter-directed (as opposed to systemic) approach, which was excluded out of our meta-analysis. In Goldhaber et al.’s research, 101 PE patients were randomized to assign the treatment (46 rt-PA, 55 heparin alone), but there were only 36 patients (18 rt-PA, 18 heparin alone) with baseline right-ventricular hypokinesis, however, in which the major bleeding events of the subgroup were not clearly stated. These might be the reasons for the different evaluation result of the incidence of major bleeding events. Another meta-analysis involved thrombolytic therapy for patients with acute submassive PE, and the authors reported that adjunctive thrombolytic therapy did not significantly reduce the risk of mortality or recurrent PE, but did prevent clinical deterioration without increasing the risk of major bleeding (35). Although these authors presented results similar to ours regarding clinical deterioration and bleeding risks, we were able to show a survival advantage in the thrombolysis group despite the absence of a significant difference in the mortality rate between the thrombolysis and anticoagulation groups (P=0.08). Similarly, recurrent PE in the thrombolytic group was significantly lower than that in the anticoagulation group, in our study (P=0.01). A possible reason for this conflict was that the meta-analysis of Nakamura et al. (35) did not include the Moderate Pulmonary Embolism Treated with Thrombolysis (MOPETT) trial (23). Our meta-analysis included all RCTs, published to date, comparing thrombolysis and anticoagulation treatment, administered via a peripheral vein, in intermediate-risk PE patients. The results of the study might help doctors to reconsider the existing clinical debate.
Bleeding risk assessments and reducing bleeding are always important considerations when adding fibrinolytic therapy to the treatment of intermediate-risk PE patients. Apart from the contraindications, increasing age (25,33,36), larger body mass index (36), and underweight (37) are associated with a higher risk of bleeding complications. On the other hand, the thrombolytic drug dose might also correlate with bleeding risk. One study that compared high-and low-dose streptokinase (SK) for the acute treatment of intermediate-risk PE patients showed similar efficacies and a reduced bleeding tendency for the lower dose SK treatment. Compared with anticoagulation, low-dose SK rapidly improved mPAP and pulmonary vascular resistance and was as safe as heparin, with regard to bleeding (26). These findings agreed with the results of a meta-analysis of studies involving a low-dose rt-PA for the treatment of acute PE (38). The MOPETT trial reported that low-dose tPA for intermediate-risk PE reduced the risk of bleeding and retained the benefits compared with anticoagulation (23). The low-dose regime was effective and safe because the lungs were the only organ receiving the entire cardiac output. All venous flow and all administered thrombolytic molecules reach the lungs, which are uniquely sensitive to thrombolysis (23). Thus, thrombolysis is warranted for the treatment of intermediate-risk PE patients, if the treatment is not contraindicated and the patients carry low risks of bleeding. Additionally, a low-dose regimen might be an effective choice.
Limitations
This meta-analysis has some limitations. First, in most of the included studies, RVD was diagnosed by echocardiography, which is not the gold standard method for risk stratification and is operator-dependent, increasing the potential bias. Further, these studies evaluated different thrombolytic and anticoagulation regimens, different outcome indicators, and different follow-up durations, restricting the extracted data. Second, only one study reported chronic thromboembolic pulmonary hypertension and recurrent PE during a 6-month follow-up period (22). Thus, we were unable to evaluate the long-term efficacy and safety of thrombolysis in the target patient population. Third, two of the seven trials were not double-blinded (19,23). However, a sensitivity analysis showed that this did not have an influence on the meta-analysis results.
Conclusions
In summary, our results showed that thrombolytic therapy for intermediate-risk PE patients, without contraindications, may reduce clinical deterioration and recurrent PE, improve RV function and pulmonary hypertension, and is associated with a trend towards a decreased 30-day, all-cause mortality. Although the total bleeding risk was higher in the thrombolytic group, the differences in the risk of major bleeding risk events were not significant between the two groups.
Acknowledgements
Funding: This study was supported by the following funding sources: China Key Research Projects of the 12th National Five-Year Development Plan (2011BAI11B00).
Authors’ contributions: Q Xu and C Wang designed the research. Q Xu and K Huang searched the database, reviewed abstracts and full text articles, extracted data, performed the statistical analysis, and drafted the initial manuscript. Z Zhai and Y Yang conducted the literature search and participated in the study design. Z Zhai, J Wang, and C Wang contributed to the critical revision of the manuscript. All authors read and approved the final manuscript. C Wang had primary responsibility for the final content.
Disclosure: The authors declare no conflict of interest.
References
- Vázquez FJ, Posadas-Martínez ML, Vicens J, et al. Incidence rate of symptomatic venous thromboembolic disease in patients from a medical care program in Buenos Aires, Argentina: a prospective cohort. Thromb J 2013;11:16. [PubMed]
- Naess IA, Christiansen SC, Romundstad P, et al. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost 2007;5:692-9. [PubMed]
- Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol 2012;25:235-42. [PubMed]
- Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost 2007;98:756-64. [PubMed]
- Sekhri V, Mehta N, Rawat N, et al. Management of massive and nonmassive pulmonary embolism. Arch Med Sci 2012;8:957-69. [PubMed]
- Mehta N, Sekhri V, Lehrman SG, et al. Management of massive and submassive pulmonary embolism. Am J Ther 2013;20:664-75. [PubMed]
- Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e152S-84S.
- Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 2008;29:2276-315. [PubMed]
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011;123:1788-830. [PubMed]
- Dong BR, Hao Q, Yue J, et al. Thrombolytic therapy for pulmonary embolism. Cochrane Database Syst Rev 2009;CD004437. [PubMed]
- Wan S, Quinlan DJ, Agnelli G, et al. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation 2004;110:744-9. [PubMed]
- Hamel E, Pacouret G, Vincentelli D, et al. Thrombolysis or heparin therapy in massive pulmonary embolism with right ventricular dilation: results from a 128-patient monocenter registry. Chest 2001;120:120-5. [PubMed]
- Worster A, Smith C, Silver S, et al. Evidence-based emergency medicine/critically appraised topic. Thrombolytic therapy for submassive pulmonary embolism? Ann Emerg Med 2007;50:78-84. [PubMed]
- Bilello KL, Murin S. Counterpoint: should systemic lytic therapy be used for submassive pulmonary embolism? No. Chest 2013;143:299-302. [PubMed]
- Ramakrishnan N. Thrombolysis is not warranted in submassive pulmonary embolism: a systematic review and meta-analysis. Crit Care Resusc 2007;9:357-63. [PubMed]
- Thabut G, Logeart D. Thrombolysis for pulmonary embolism in patients with right ventricular dysfunction: con. Arch Intern Med 2005;165:2200-3; discussion 2204-5. [PubMed]
- Jiménez D. Point: should systemic lytic therapy be used for submassive pulmonary embolism? Yes. Chest 2013;143:296-9. [PubMed]
- Goldhaber SZ. Thrombolytic therapy for patients with pulmonary embolism who are hemodynamically stable but have right ventricular dysfunction: pro. Arch Intern Med 2005;165:2197-9; discussion 2204-5. [PubMed]
- Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993;341:507-11. [PubMed]
- Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med 2002;347:1143-50. [PubMed]
- Becattini C, Agnelli G, Salvi A, et al. Bolus tenecteplase for right ventricle dysfunction in hemodynamically stable patients with pulmonary embolism. Thromb Res 2010;125:e82-6. [PubMed]
- Fasullo S, Scalzo S, Maringhini G, et al. Six-month echocardiographic study in patients with submassive pulmonary embolism and right ventricle dysfunction: comparison of thrombolysis with heparin. Am J Med Sci 2011;341:33-9. [PubMed]
- Sharifi M, Bay C, Skrocki L, et al. Moderate pulmonary embolism treated with thrombolysis (from the "MOPETT" Trial). Am J Cardiol 2013;111:273-7. [PubMed]
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014;12:459-68. [PubMed]
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402-11. [PubMed]
- Abdelsamada AA, El-Morsib AS, Mansourb AE. Efficacy and safety of high dose versus low dose streptokinase for treatment of submassive pulmonary embolism. Egyptian Heart Journal 2011;63:67-72.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1. 0 [updated March 2011]. The Cochrane Collaboration, 2011.
- Konstantinides S, Geibel A, Olschewski M, et al. Association between thrombolytic treatment and the prognosis of hemodynamically stable patients with major pulmonary embolism: results of a multicenter registry. Circulation 1997;96:882-8. [PubMed]
- Kline JA, Steuerwald MT, Marchick MR, et al. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest 2009;136:1202-10. [PubMed]
- Fei J, Tang Y, Wu J, et al. Thrombolytic and anticoagulant therapy for acute submassive pulmonary embolism. Exp Ther Med 2014;7:103-108. [PubMed]
- Perlroth DJ, Sanders GD, Gould MK. Effectiveness and cost-effectiveness of thrombolysis in submassive pulmonary embolism. Arch Intern Med 2007;167:74-80. [PubMed]
- Cao Y, Zhao H, Gao W, et al. Systematic review and meta-analysis for thrombolysis treatment in patients with acute submassive pulmonary embolism. Patient Prefer Adherence 2014;8:275-82. [PubMed]
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014;311:2414-21. [PubMed]
- Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014;129:479-86. [PubMed]
- Nakamura S, Takano H, Kubota Y, et al. Impact of the efficacy of thrombolytic therapy on the mortality of patients with acute submassive pulmonary embolism: a meta-analysis. J Thromb Haemost 2014;12:1086-95. [PubMed]
- Mikkola KM, Patel SR, Parker JA, et al. Increasing age is a major risk factor for hemorrhagic complications after pulmonary embolism thrombolysis. Am Heart J 1997;134:69-72. [PubMed]
- Wang C, Zhai Z, Yang Y, et al. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest 2010;137:254-62. [PubMed]
- Zhang Z, Zhai ZG, Liang LR, et al. Lower dosage of recombinant tissue-type plasminogen activator (rt-PA) in the treatment of acute pulmonary embolism: a systematic review and meta-analysis. Thromb Res 2014;133:357-63. [PubMed]


