One-stage hybrid procedure without sternotomy for treating thoracic aortic pathologies that involve distal aortic arch: a single-center preliminary study
Introduction
Over the last 10 years, thoracic endovascular aortic repair (TEVAR) has been widely used to treat thoracic aortic pathologies. TEVAR is an effective but less invasive strategy. The left subclavian artery (LSA) and the left common carotid artery (LCCA) may be covered to achieve good fixation of stent grafts in cases that involve distal aortic arch. However, covering the origins of these arteries may result in several complications, such as left upper limb ischemia, posterior circulation ischemia, and even cerebral infarction. Therefore, reconstructing supra-arch branch vessels is necessary to achieve desirable outcomes. Strategies that include replacing the aortic arch with a frozen elephant stent and a hybrid operation (TEVAR combined with carotid or axillary artery bypass) have been employed recently to treat distal arch pathologies. However, the optimal treatment for descending aortic disease that involves distal aortic arch remains uncertain. Based on our experience, we retrospectively evaluated the effectiveness of the one-stage hybrid procedure for treating thoracic aortic pathologies that involve distal aortic arch.
Materials and methods
Patients
A series of consecutive cases involving 45 patients with descending aortic disease underwent the aforementioned hybrid procedure in our center from April 2009 to August 2014. The subjects included 35 male and 10 female patients, with a mean age 62.49±8.63 years (range, 42-77 years). The clinical entities included 19 Stanford type-B aortic dissections, 8 penetrating aortic ulcers, 9 aortic arch aneurysms, 5 pseudoaneurysms of the aortic arch, and 4 proximal type-I endoleak after TEVAR of the aortic dissection. The clinical data of the patients are provided in Table 1.
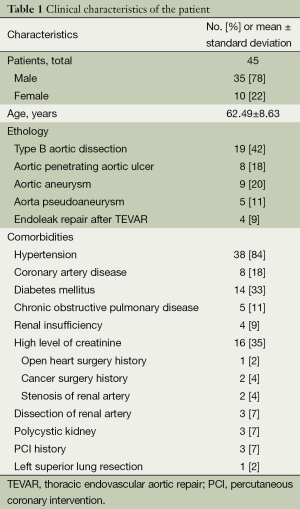
Full table
This study has been approved by the Institutional Review Board of Anzhen Hospital, which is affiliated with Capital Medical University (Beijing, China). A signed informed consent was obtained from each patient involved in this study.
Prosthetic grafts and stent grafts
A total of 45 GORE-TEX® prosthetic grafts (W. L. Gore & Associates, Inc., Flagstaff, AZ, USA) and 45 plugs (P1416, Huayishengjie Co., Beijing, China) were used in all the patients.
Meanwhile, four stent graft systems were employed in this study: Zenith TX2 (Cook, Inc., Lunderquist, Denmark) in 29 cases, Talent (Medtronic, Inc., Minneapolis, USA) in two cases, Grikin (Grikin Advanced Materials Co., Ltd., Beijing, China) in eight cases, and Hercules (MicroPort Co., Ltd., Shanghai, China) in three cases. In total, 49 stent grafts were used.
Hybrid procedure
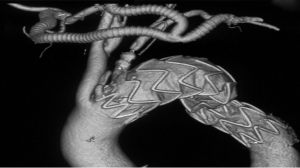
The one-stage hybrid procedure was performed in a hybrid operating room by a team that consisted of a cardiovascular surgeon, an interventional cardiologist, a radiologist, and an anesthetist. After general anesthesia was administered, the dissociation of the double axillary artery from an incision below the collarbone or the dissociation of the LCCA from an incision at the front edge of the sternocleidomastoid was performed depending on the type of pathology. We created a channel from the skin incision for the left axillary artery to the skin incision for the right axillary artery and the skin incision of the common carotid artery. We then dissociated the femoral artery. Subsequently, heparin (1 mg/kg or 125 IU/kg) was administered intravenously. A GORE-TEX® prosthetic graft with a diameter of 8 mm was anastomosed to the right axillary artery via end-to-side anastomosis if the disease only involved the LSA. However, if the disease involved the LCCA, a “T” GORE-TEX® prosthetic graft with a diameter of 8 mm was initially anastomosed to the right axillary artery via end-to-side anastomosis, and then another branch was anastomosed to left axillary artery also via end-to-side anastomosis. The LCCA was transected circumferentially at 5 to 10 mm distal to its origin. The proximal segment of the LCCA was sutured, and then end-to-end anastomosis was performed between the third branch of the prosthetic graft and the distal part of the common carotid artery. The embolization (P1416, Huayishengjie Co., Beijing, China) of the proximal LSA was performed via the left axillary artery (Figure 1). The bypass graft was located in the upper part of the sternum in case of open heart surgery. The angiography of the aortic arch revealed that the prosthetic graft was patent, and a satisfactory healing process with complete thrombosis in the proximal part of the LSA was observed.
Postoperative care
All of the patients were transferred to the intensive care unit after the hybrid procedure. Their systolic blood pressure was controlled to less than 120 mmHg by using intravenous drugs. Respiration assistance was withdrawn after a patient became fully awake. Oral medicine was given to control blood pressure. An intravenous antibiotic was used for three postoperative days. No anticoagulant was administered. Postoperative clinical features are in Table 2.
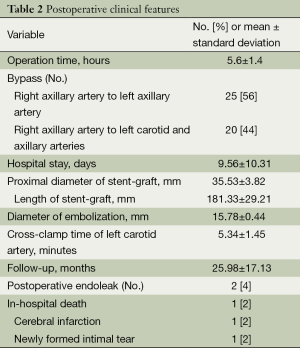
Full table
Follow-up
Computed tomography (CT) scans were performed before the patients were discharge from the hospital, as well as 3, 6 months, and annually postoperative for follow-up.
Statistical analysis
Clinical data were collected from a database. All data used in this study were expressed as mean ± SD.
Results
In-hospital results
The postoperative results are provided in Table 2. The technical success rate was 95.6% (43/45). We observed no incidence of paraplegia and renal failure. The median duration of hospital stay was 9.56±10.31 days, and the average operation time was 5.6±1.4 hours. The pathologies of 43 of the patients were excluded completely. Two cases of Stanford type-B aortic dissection exhibited type-I endoleak. After the stent grafts were deployed, an angiography showed that a small amount blood was still entering the false lumen through the primary tear. Another stent graft was added at the proximal end of the first stent graft to eliminate the type-I endoleak. This time, the angiography showed that the amount of blood entering the false lumen was reduced. We conducted follow-up with the patients religiously. After 3 months, endoleak was still observed in the two patients, who were then admitted to our center for open surgery.
One case of mortality was recorded a day after the hybrid procedure. The patient who had aortic aneurysm died of a sudden drop in blood pressure.
Meanwhile, one patient suffered from cerebral infarction after the operation. Hemiplegia of the right limbs was observed in this patient after the effects of the general anesthesia dissipated. The CT scan revealed that cerebral infarction was the cause of hemiplegia. After 3 days, the patient was transferred to the rehabilitation unit for recovery. The patient remained bedridden and could not move on her own until October 2014.
The 41 patients who exhibited no complication were discharged.
Follow-up results
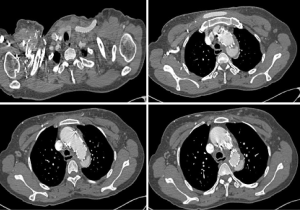
Clinical data were obtained through personal and telephone interviews with the patients and their family members. Follow-up was conducted for all the patients from the 2nd to the 65th month postoperative (mean, 26.0±17.1). In the two cases with endoleak, the results of CT scans showed that the endoleak (Figure 2) remained until the 3rd month of follow-up although no chest pain or other symptoms was reported. The patients in both cases were admitted to our center for surgical intervention. A newly formed intimal tear in the distal part of the stent graft was observed in one patient, which caused blood to return to the false lumen at the stent graft level. This patient underwent a second TEVAR procedure. Another stent graft was added at the distal end of the first stent graft, and the tear was sealed completely. No mortality or stenosis of prosthetic grafts was observed during follow-up.
Discussion
Since the introduction of endovascular repair by Volodos et al. (1) and Dake et al. (2), TEVAR has been widely used to treat several thoracic aortic diseases. The key factor in the success of TEVAR is the ideal proximal landing zone. However, the efficiency of TEVAR is limited in descending thoracic aortic pathologies that involve distal aortic arch because it results in an inadequate proximal landing zone. In 2003, we developed a one-stage open procedure for arch pathologies that involved total arch replacement using frozen elephant trunk implant; although this procedure obtained good results, it could lead to hypothermic cardiac arrest, extracorporal circulation, and selective cerebral perfusion (3). Moreover, this open procedure was accompanied by a high risk of mortality as well as risks of paraplegia and cerebral stroke (4). Consequently, debranching and surgical supra-arch branch vessel bypass combined with TEVAR (5) emerged as an important strategy to expand landing zones and simultaneously prevent complications, such as left upper limb ischemia, posterior circulation ischemia, and even cerebral infarction. In our center, open surgery is performed if a pathology involves all of the aortic arches. However, if the pathology only involves the proximal arch, then surgical supra-arch branch vessel bypass combined with TEVAR is an appropriate alternative technique, which is potentially less invasive and produces lower mortality (6). This procedure has increased endovascular treatment options (7).
The surgical supra-arch branch vessel bypass procedure was primarily developed as a treatment for obliterative diseases. Afterward, this procedure was determined as capable of treating more thoracic aortic pathologies (8). This procedure is technically easy to perform and can be completed rapidly. It does not require opening the chest of patients, and thus, pulmonary complications caused by median sternotomy are avoided. Other potential advantages include eliminating the risk of bleeding from aortic anastomosis, hypothermia-related coagulopathy, as well as ischemic injury to the nervous system and visceral organs resulting from prolonged circulatory arrest (8).
During surgical supra-arch branch vessel bypass, we ligated the proximal LCCA and sealed the proximal LSA with a plug. No main branch existed in the proximal part of the LCCA, and thus, abscission and ligation of this artery blocked blood from flowing back to the space between the aortic wall and the stent graft. Ligation of the proximal part of the left axillary artery was avoided because the left vertebral artery originated from the proximal part of the LSA. Blood flow into the left vertebral artery would be blocked if the proximal part of the left axillary artery was ligated. Ischemia of the left vertebral artery would result in posterior circulation ischemia and even cerebral infarction (9,10).
In our study, we found that plug implantation in the proximal LSA prevented the potential occurrence of endoleak and preserved the left vertebral artery.
This treatment was initially administered only in patients with delicate conditions, particularly those with serious comorbidities. The bypass grafts were all patent during follow-up, and no blockage or stenosis was observed. We speculated that the ligation of the proximal LCCA and the sealing of the proximal LSA, which directed blood flow into the LCCA and the LSA, contributed to the aforementioned condition of the bypass graft. The fast velocity of blood from the bypass graft would not be conducive to thrombus formation inside the bypass graft. Thus, we believe that ligating the proximal LCCA and sealing the proximal LSA will prevent stenosis of the bypass graft.
In the current study, one patient suffered from cerebral infarction. The stroke rate was 2% (1 out of 45), which was lower than reported (11-14). Arteriosclerosis was discovered in the LCCA of this patient. In addition, the LCCA was difficult to clamp. Thus, we believed that the plaque in the LCCA flowed into the cerebrovascular, which resulted in cerebral infarction. This patient underwent tracheotomy 1 week after the hybrid procedure and became bedridden since then. She receives nasal feeding every day.
Another patient died of a sudden drop in blood pressure 1 day after the procedure. Ultrasound showed massive left pleural effusion. Thus, we speculated that the cause of death was aortic rupture. In our center, in-hospital mortality was 4% (n=2) for the hybrid procedure and 6.27% for surgical arch replacement (15).
After the bypass, the blood passing through the LCCA and the LSA was supplied by the innominate artery via the bypass graft. No dysperfusion of the brain or the arms was observed in all the patients. Thus, we maintained that blood flow from the innominate artery was adequate to supply the brain and the two arms. Renal failure that required dialysis was reported at 5.7% and 3.8% in the arch debranching group and the elephant trunk group, respectively (16). No renal dysfunction was reported except in two patients with elevated creatinine levels, although dialysis was not required. This result may be attributed to the procedure being less invasive.
During the follow-up period, all the patients underwent CT scans at the 3rd, 6th month, and 1st year postoperatively.
Early endoleak is a serious complication of TEVAR, with a rate ranging from 7% to 42% (17-19). Inadequate proximal sealing zone has been reported as the main cause of failure, excluding entry tear or aneurysmal sac (20). In our series of cases, a lower endoleak rate of 4% (2 out of 45) was observed. Endoleak occurred in two aortic dissection cases. These two patients were closely monitored, but the endoleak remained. The CT scan results of the two patients revealed that the tears were large and localized at the outer curvature of the aortic arch. The location of the tear may be the risk factor for endoleak. Xu et al. (21) reported that endoleak was the main cause of death after TEVAR during the follow-up period. The two patients in our study underwent open surgery in our center, and they recovered well after the surgery. Based on our experience, the hybrid technique is not recommended for type-B aortic dissection when the tear is located at the greater curvature; open surgery is more appropriate for such cases. Meanwhile, a newly formed intimal tear was observed in one patient during follow-up. The symptoms of the tear, as reported by the patient, included occasional back pain. The result of the CT scan showed that the diameter of the descending aorta was bigger than before. The dilatation of the distal part of the stent graft caused the tear. The same complication has been reported by other authors (22). We then placed another stent graft at the distal part of the first stent graft. During the follow-up period, the back pain of the patient disappeared and the tear excluded completely.
Spinal cord ischemia is one of the severe complications of TEVAR.
A EUROSTAR registry reported that the observed risk factors for spinal cord ischemia include covering of the LSA without revascularization, renal failure, concomitant open abdominal aortic surgery, and the use of three or more stent grafts. This paper has also reported that subclavian artery revascularization is the most important factor in spinal protection (23). In our center, spinal cord ischemia is rarely encountered because the LSA is reconstructed by bypass or the chimney technique (24) if it needs to be covered.
The hybrid procedure is unsuitable for patients whose left vertebral artery originates from the aortic arch. In such cases, a stent graft will block the vertebral artery. Thus, the location of the left vertebral artery should be determined before the hybrid procedure can be performed.
The primary limitation of this work is obvious. As a retrospective study, the comparison between supra-arch branch bypass combined with endograft implantation (i.e., the hybrid procedure) group and the open surgery group was not performed randomly. The preliminary result of the hybrid technique for treating thoracic aortic pathologies that involved distal aortic arch was satisfactory. However, evaluating whether this technique can improve long-term outcome, such as the absence of endoleak and stenosis of the bypass graft, is necessary.
Conclusions
The initial results obtained from this study suggest that the one-stage hybrid procedure is a suitable therapeutic option for thoracic aortic pathologies that involve distal aortic arch. However, this hybrid procedure is not recommended for type-B aortic dissection, i.e., when the tear is located in the greater curvature or near the LSA, because of a high possibility of endoleak occurrence. The ligation of the proximal LCCA and the sealing of the proximal LSA made the bypass graft patent. However, these promising initial results must be verified through comparative studies with a larger number of open surgery cases and longer follow-up periods.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Volodos NL, Karpovich IP, Troyan VI, et al. Clinical experience of the use of self-fixing synthetic prostheses for remote endoprosthetics of the thoracic and the abdominal aorta and iliac arteries through the femoral artery and as intraoperative endoprosthesis for aorta reconstruction. Vasa Suppl 1991;33:93-5. [PubMed]
- Dake MD, Miller DC, Semba CP, et al. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med 1994;331:1729-34. [PubMed]
- Sun L, Qi R, Zhu J, et al. Total arch replacement combined with stented elephant trunk implantation: a new "standard" therapy for type a dissection involving repair of the aortic arch? Circulation 2011;123:971-8. [PubMed]
- Higashi R, Matsumura Y, Yamaki F. A single stage hybrid repair of a complicated acute type B dissection with aortic arch involvement. Ann Vasc Dis 2014;7:141-4. [PubMed]
- Kuratani T, Sawa Y. Current strategy of endovascular aortic repair for thoracic aortic aneurysms. Gen Thorac Cardiovasc Surg 2010;58:393-8. [PubMed]
- Byrne J, Darling RC 3rd, Roddy SP, et al. Long term outcome for extra-anatomic arch reconstruction. An analysis of 143 procedures. Eur J Vasc Endovasc Surg 2007;34:444-50. [PubMed]
- Schoder M, Lammer J, Czerny M. Endovascular aortic arch repair: hopes and certainties. Eur J Vasc Endovasc Surg 2009;38:255-61. [PubMed]
- Czerny M, Fleck T, Zimpfer D, et al. Combined repair of an aortic arch aneurysm by sequential transposition of the supra-aortic branches and endovascular stent-graft placement. J Thorac Cardiovasc Surg 2003;126:916-8. [PubMed]
- Chung J, Kasirajan K, Veeraswamy RK, et al. Left subclavian artery coverage during thoracic endovascular aortic repair and risk of perioperative stroke or death. J Vasc Surg 2011;54:979-84. [PubMed]
- Manninen H, Tulla H, Vanninen R, et al. Endangered cerebral blood supply after closure of left subclavian artery: postmortem and clinical imaging studies. Ann Thorac Surg 2008;85:120-5. [PubMed]
- Cao P, De Rango P, Czerny M, et al. Systematic review of clinical outcomes in hybrid procedures for aortic arch dissections and other arch diseases. J Thorac Cardiovasc Surg 2012;144:1286-300,1300.e1-2.
- Geisbüsch P, Kotelis D, Müller-Eschner M, et al. Complications after aortic arch hybrid repair. J Vasc Surg 2011;53:935-41. [PubMed]
- Vallejo N, Rodriguez-Lopez JA, Heidari P, et al. Hybrid repair of thoracic aortic lesions for zone 0 and 1 in high-risk patients. J Vasc Surg 2012;55:318-25. [PubMed]
- Antoniou GA, Mireskandari M, Bicknell CD, et al. Hybrid repair of the aortic arch in patients with extensive aortic disease. Eur J Vasc Endovasc Surg 2010;40:715-21. [PubMed]
- Sun LZ, Ma WG, Zhu JM, et al. Sun's procedure for chronic type A aortic dissection: total arch replacement using a tetrafurcate graft with stented elephant trunk implantation. Ann Cardiothorac Surg 2013;2:665-6. [PubMed]
- Moulakakis KG, Mylonas SN, Markatis F, et al. A systematic review and meta-analysis of hybrid aortic arch replacement. Ann Cardiothorac Surg 2013;2:247-60. [PubMed]
- Dong ZH, Fu WG, Wang YQ, et al. Retrograde type A aortic dissection after endovascular stent graft placement for treatment of type B dissection. Circulation 2009;119:735-41. [PubMed]
- Palma JH, de Souza JA, Rodrigues Alves CM, et al. Self-expandable aortic stent-grafts for treatment of descending aortic dissections. Ann Thorac Surg 2002;73:1138-41; discussion 1141-2. [PubMed]
- Lotfi S, Clough RE, Ali T, et al. Hybrid repair of complex thoracic aortic arch pathology: long-term outcomes of extra-anatomic bypass grafting of the supra-aortic trunk. Cardiovasc Intervent Radiol 2013;36:46-55. [PubMed]
- Pamler RS, Kotsis T, Görich J, et al. Complications after endovascular repair of type B aortic dissection. J Endovasc Ther 2002;9:822-8. [PubMed]
- Xu SD, Huang FJ, Yang JF, et al. Early and midterm results of thoracic endovascular aortic repair of chronic type B aortic dissection. J Thorac Cardiovasc Surg 2010;139:1548-53. [PubMed]
- Lopera J, Patiño JH, Urbina C, et al. Endovascular treatment of complicated type-B aortic dissection with stent-grafts:: midterm results. J Vasc Interv Radiol 2003;14:195-203. [PubMed]
- Buth J, Harris PL, Hobo R, et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: Incidence and risk factors. a study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) registry. J Vasc Surg 2007;46:1103-10; discussion 1110-1. [PubMed]
- Xue Y, Sun L, Zheng J, et al. The chimney technique for preserving the left subclavian artery in thoracic endovascular aortic repair. Eur J Cardiothorac Surg 2015;47:623-9. [PubMed]


