Switching off malignant pleural effusion formation—fantasy or future?
Introduction
Malignant pleural effusion (MPE), defined by the identification of malignant cells or tumor tissue in the pleural space, is a severe medical issue as it underlines advanced malignancy. MPE is a distinct form of metastatic disease; about 80% of MPEs are caused by pleural metastases originating from adenocarcinomas of the lung, breast and ovary or by lymphomas. Mesothelioma is the commonest type of primary pleural tumor and is associated with MPE in more than 90% of cases (1-3). MPE affects 500-700 people per million population every year, an incidence similar to that of lung cancer (4,5).
The incidence of MPE and the associated healthcare costs are expected to rise in the future as the lifespan of cancer patients increases. Despite the progress in cancer treatments, MPE remains refractory to current treatment approaches and is associated with high morbidity and mortality. The life expectancy depends on the origin of the primary tumor and is approximately 4 months for lung cancer patients (2). The most common presenting symptoms of MPE are dyspnea, cough, fatigue, weight loss and pleuritic pain (6). These symptoms often impact on quality of life. There is thus an urgent need for better treatment for MPE.
Current management of MPE
The ideal management of MPE would effectively control fluid reaccumulation and provide long-term relief of patient symptoms and distress, have limited side effects, improve quality of life, be well tolerated, be minimally invasive, incur low healthcare costs and can be hence delivered on an outpatient basis. Current treatments fail to fulfil the above criteria and merely offer palliation of symptoms, without improving life expectancy let alone offering a cure. The aim of currently-available treatment for MPEs is to optimize respiratory function and quality of life.
Thoracentesis
Therapeutic thoracentesis is often the first approach in the management of MPE to determine the symptomatic benefits from fluid removal. Thoracentesis avoids hospitalization and can provide short-term symptom relief; however pleural fluid and related symptoms recur in many patients within 30 days (7). A considerable number of patients does not benefit from thoracentesis because dyspnea is often caused by concomitant morbidities (8,9). Complications from thoracentesis include pneumothorax, vasovagal reactions, cough, infections and chest pain. Repeated thoracentesis should only be applied to those of poor general condition and short life expectancy, those with a slow rate of fluid accumulation or those unsuitable for pleurodesis or indwelling catheters (2,7,10).
Pleurodesis
Pleurodesis is usually the method of choice in the management of recurrent symptomatic MPE (11). Patient selection is critical for successful pleurodesis. Improvement of symptoms from thoracentesis and a life expectancy >2-3 months are often advocated for patient selection for pleurodesis (6,12). However, no accurate clinical or biochemical criteria exist to help select suitable candidates (13).
Pleurodesis aims at obliterating the pleural space to prevent the fluid accumulation. The procedure involves mechanical- or chemical-induced pleural inflammation, resulting in pleural fibrosis and fusion of the visceral and parietal pleura (5). Chemical pleurodesis employs the instillation of a chemical irritant into the pleural space. A number of sclerosing agents have been cited in the literature including talc (considered most effective) (2,7,11,14,15), chemotherapeutic drugs, biologic agents, antibiotics and recently silver nitrate and povidone iodide (6,14-18).
Common side effects from pleurodesis include pain and fever, but serious complications of reexpansion edema, persistent air leak, acute respiratory distress, pneumonitis, local infections and hypotension can occur (10). Pleurodesis is also associated with prolonged hospitalization and significant healthcare costs (3) and does not guarantee permanent control of fluid reaccumulation. Studies have shown that fluid reaccumulation occurs in 36% of patients by day 17 post-pleurodesis (19). This figure increases to 50% of patients at 6 months after pleurodesis, despite guideline implementation (20). In one study, 19% of patients with successful pleurodesis died after a median time of 61 days (19). Better prognostic predictors for successful pleurodesis are needed.
Indwelling pleural catheters (IPCs)
IPCs allow ambulatory drainage of fluid according to patients’ symptoms. Insertion of indwelling catheters is accepted as a second-line treatment when pleurodesis is not recommended or failed. However, recent studies indicate that indwelling catheters provide the same benefits as pleurodesis in controlling patients’ symptoms with significantly less hospitalization time (21-23). Over 90% of patients remain asymptomatic for at least 30 days (24) following IPC insertion. Furthermore, up to 70% of patients with IPCs can develop spontaneous pleurodesis (25); otherwise sclerosants can be instilled through the catheter. IPCs provide efficient symptom control with minimal invasion, and provide an excellent tool for longitudinal clinical research. Placement is simple and safe and can often be performed as a day case with local anesthesia. Complications from IPCs, including infections, clogging or dislodgement of the catheter, are generally well tolerated by patients and easily managed (22,26-28).
Rationale for targeted therapies against MPE
Successful treatment of MPE represents an ongoing challenge in clinical practice. Because of the inadequacies of thoracentesis, pleurodesis and IPCs, additional therapeutic approaches have been tried, including surgery, systemic therapy, gene therapy, chemotherapy and intrapleural immunotherapy (29,30). This plethora of management options however has not shown proven benefits. Pleurodesis for example does not treat the primary tumor and offers merely transient relief of MPE-associated breathlessness. Chemotherapy can be employed in certain occasions as a first line treatment for the primary tumor, however many tumors, e.g., mesothelioma, are chemoresistant (31) and many patients are not fit for aggressive chemotherapy. Moreover, talc pleurodesis is associated with long hospitalization time as it has been shown that the patients can spend up to 6.1% of their remaining lifespan in the hospital during the procedure and a median of 18 days in hospital including subsequent admissions (22,32). Discomfort and morbidity from MPE constitute an additional human cost for these patients.
Development of novel, effective, personalized treatment for individual patients is impaired not only by the lack of reliable predictors of patient outcome and survival but also by our incomplete understanding of basic biologic aspects of cancer metastasis to the pleural space and effusion development. For example, current treatment for lung cancer is guided by mutation status of key oncogenes like epidermal growth factor receptor (EGFR) or Kirsten rat sarcoma viral oncogene homolog (KRAS), a downstream factor of the EGFR pathway. Recent studies suggest that tumor cells metastasizing to the pleural space constitute a cell population disparate to the primary tumor, as evidenced by the significant discordance in EGFR mutation status between the primary tumor and cancer cells in the effusion (33). These findings suggest that MPE is not merely a complication of the primary malignancy but could represent a different cancer phenotype. This notion in turn raises new important questions in the field of MPE. The possible genetic and phenotypic differences between cancer cells from the effusions and from the primary neoplasms should be addressed. Expression profiles of cancer cells in effusions should be unveiled which would be a crucial factor in guiding therapeutic decisions and monitoring tumor response. Answers to these questions will likely result in therapeutic innovations for patients with MPE.
A key area of focus for MPE research should be to develop drugs targeting fluid formation, taking however into consideration the heterogeneity of the various tumors and how it can impact drug efficacy. A thorough understanding of the pathobiology of MPE is key to the development of novel therapy specifically targeting mechanisms that promote the production of MPE.
Current concepts in MPE pathobiology
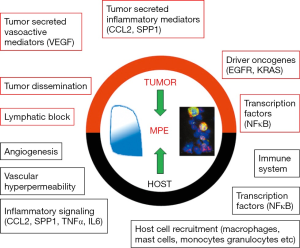
Pleural fluid normally enters the pleural space through the systemic capillaries of both the parietal and the visceral pleura and is removed by lymphatic drainage through the stomata of the parietal pleura, by absorptive pressure gradients through the parietal pleura and by cellular mechanisms (34). A well-established mechanism in the pathogenesis of MPE is attributed to tumor-associated blockade of local lymphatic outflow resulting in the impairment of effective pleural fluid absorption (35). The blockade of the lymphatic network is observed due to tumor dissemination in the parietal pleural stomata and mediastinal lymph nodes, thus obstructing drainage of the fluid from the pleural cavity (36). However, blocking fluid removal from the pleural cavity cannot be the only cause leading to MPE formation, as it has been shown that even though many tumors cause lymphatic obstruction, not all of them induce MPE formation (35,36). These recent findings have challenged the classic view for MPE pathogenesis and have demonstrated that MPE formation constitutes a complex biological phenomenon incorporating interactions between tumor cells and the host vasculature, the immune system and other host cells in the pleural microenvironment (Figure 1) (37).
Increased vascular permeability leading to enhanced fluid production through excessive plasma leakage is now a well-documented event observed with MPE (38). Recent studies have highlighted the role of pleural tumor foci in rendering the pleural vessels hyperpermeable. It has been shown that tumor cells secrete a surplus of several vasoactive mediators which contribute to the development of MPE by increasing blood vessel leakiness. Such mediators include vascular endothelial growth factor (VEGF) and angiopoietins (38,39). Interestingly, vasculature-associated mediators, namely endostatin, which impair MPE formation have also been described (40,41). Newer evidence incriminates host cells, in addition to tumor cells, in producing vasoactive mediators, as it has been shown that host-elaborated transforming growth factor-β (TGF-β) induces production of VEGF by mesothelial cells, thereby contributing to MPE formation (42).
A breakthrough in the study of MPE formation was accomplished by the advent of immunocompetent mouse models of MPE (43,44). These mouse models permitted the identification of additional tumor-elaborated MPE-triggering signaling molecules, which were found to be proinflammatory mediators like interleukin (IL)-6, tumor necrosis factor (TNF), chemokine ligand 2 (CCL2) and secreted phosphoprotein-1 (SPP1, also known as osteopontin) (45-48). These molecules promote MPE formation in more than one way, as they are potent inducers of vascular hyperpermeability, angiogenesis and inflammation. In addition, signaling cascades originating from the aforementioned factors result in the recruitment of host inflammatory and mesothelial cells to the pleural space which in turn secrete CCL2, SPP1, VEGF and other molecules like IL-5, further promoting MPE development (42,44,48).
Host cells attracted to the pleural space by tumor-elaborated signaling include granulocytes, monocytes, macrophages, mast cells, lymphoid cells and, possibly, other bone marrow-derived cells such as endothelial and fibroblast progenitors. These cells interact with tumor cells and likely favor the local manifestation of angiogenic and inflammatory events, important for MPE pathogenesis (29,37,47). It is becoming clear that the current view for MPE pathobiology should include tumor-host cross-talk in the pleural space which dictates the occurrence of vasoactive signaling, host cell recruitment and activation, vascular leakage, angiogenesis, inflammation and tumor dissemination and ultimately MPE formation.
Blocking angiogenesis to halt pleural fluid formation
Our improved understanding of MPE pathobiology has led to the identification of new therapeutic targets. VEGF, one of the most important factors of angiogenesis and vascular permeability, has been one of the first molecules targeted in the clinic as there is extensive evidence linking VEGF expression with tumor progression and MPE pathobiology. Many studies have demonstrated that increased expression levels of VEGF in primary tumors are associated with enhanced angiogenesis in the tumor and poor survival for patients with non-small cell lung cancer (NSCLC) and MPE (49,50). VEGF expression levels are increased in MPE irrespective of the underlying primary neoplasm, as shown in cases of MPE from metastatic breast or lung cancers as well as mesothelioma (51-53). VEGF expression levels have also been assessed as a diagnostic tool in MPE (54). Experimental mouse models have further established the important role of VEGF in MPE development. Using a nude mouse model it has been shown that VEGF expression levels, either in the MPE or after injection of the recombinant protein, correlated proportionately with vascular permeability. Pretreating the mice with a VEGF receptor (VEGFR) antibody reduced the observed increase in vascular permeability (53). Additional studies incriminated tumor-elaborated VEGF as a critical factor for the induction of pleural fluid formation, by favoring vascular hyperpermeability after injection of human non-small cell lung adenocarcinoma cell lines in nude mice (38). VEGF-mediated MPE development was also described after autocrine IL-6 induced activation of signal transducer and activator of transcription (Stat) 3 in lung adenocarcinoma (45).
Many therapeutic interventions targeting angiogenesis and vascular permeability have been undertaken against experimental MPE. Blockade of VEGF signaling by the VEGFR tyrosine kinase phosphorylation inhibitor PTK787 reduced MPE formation by inhibiting vascular permeability (55). Treatment with ZD6474 (vandetanib), another VEGFR tyrosine kinase inhibitor with some partial activity against EGFR tyrosine kinase, reduced tumor vascularization and proliferation and significantly impaired MPE development in nude mice injected with NSCLC cells (56). Bevacizumab (avastin), a humanized VEGF monoclonal neutralizing antibody, was also reported to exert promising effects when administered in high dosages in patients with malignant effusions (57). On the other hand, treatment of mice with experimental MPE from lung adenocarcinoma using temsirolimus, a drug reported to inhibit tumor angiogenesis by reducing synthesis of VEGF (58), did not curtail effusion formation (59).
The efficacy of bevacizumab in patients with NSCLC has been extensively evaluated in many clinical trials, which showed that the drug, in combination with chemotherapy, can improve survival of patients with lung adenocarcinoma (60). There are also ongoing phase II clinical trials designed to assess the effect of intrapleural administration of bevacizumab for MPE in patients with NSCLC (ClinicalTrials.gov identifiers: NCT02054052 and NCT02005120). Conversely, a recently completed clinical trial (ClinicalTrials.gov Identifier: NCT00402896) investigating the effects of ZD6474 on the volume of MPE in patients with NSCLC concluded that the patients did not benefit significantly from the treatment (61) but the effect on effusion was not defined.
Angiopoietins pose other potent regulators of MPE formation as they play critical roles in angiogenesis, tumor-associated angiogenesis, vascular permeability and inflammation (62-66). It has been shown that pleural expression levels of angiopoietin are increased in patients with MPE (67). In a mouse model of experimental MPE from lung adenocarcinoma cells, angiopoietin signaling through the tyrosine kinase receptor Tie2 was blocked by the systemic administration of a soluble form of the same receptor. The results of this study indicated that blockade of the angiopoietin/Tie2 axis reduced MPE formation, impaired pleural tumor dissemination, attenuated vascular hyperpermeability and tumor angiogenesis and decreased recruitment of inflammatory cells to the pleural cavity (39). In a recent study, an angiopoietin 2-specific inhibitor was administered in an experimental mouse model of MPE together with recombinant human endostatin. The authors concluded that the two drugs exerted synergistic effects in controlling MPE formation, tumor growth, inflammation, tumor angiogenesis and vascular hyperpermeability (68). Beneficial effects of human recombinant endostatin on experimental MPE were also reported independently (41). Further studies are needed in order to evaluate the therapeutic application of these interventions in clinical practice.
Targeting driver oncogenes against MPE
MPE accumulation appears to correlate with specific mutations which can determine the therapeutic regimen [(69); see also review article in this issue by Agalioti et al.]. Clinical data show the presence of different mutations in pleural metastatic sites compared to the primary tumors (37). For example, EGFR mutations are more common in the pleural fluid whereas KRAS mutations are more frequent in primary tumors (50,70).
There is potential for treatment of MPE to be personalized according to the mutation status of the pleural tumor cells identified by DNA analysis, which may have prognostic implications. Identifying the driver mutations may open up possibilities of new treatments. When such mutations are not found, a more general chemotherapy protocol can be predilected. Particular mutations of the EGFR gene can induce growth and spread of the tumor. In these cases, targeted tyrosine kinase inhibitors (TKIs), like erlotinib and gefitinib can be used as an alternate approach to chemotherapy (71). Several studies indicate that EGFR positive pleural metastases have a similar response to TKI therapy as seen in other EGFR positive metastatic sites. EGFR gene exon 19 deletions, exon 21 mutation L858R, exon 21 mutation L861Q and exon 18 mutation at amino acid location 719 are the most common mutations associated with increased response to TKIs (72,73). The most common EGFR mutations associated with lack of response or resistance to EGFR TKIs are exon 20 mutation T790M, exon 20 mutation S768I, and identified insertions in exon 20 (74). Unfortunately, almost all patients who initially respond to an EGFR-TKI subsequently develop resistance and experience progression of the disease. Secondary mutations in EGFR (T790M, D761Y, and L747S) appear to be responsible for the majority of cases of acquired resistance to EGFR-TKIs (75,76).
The rat sarcoma (RAS) oncogene product is considered a major target for anticancer therapy. Our group has studied the role of aminobiphosphonates, such as zoledronic acid (ZA), in an experimental model of MPE, as this drug class is known to exert indirect antitumor effects. In experimental MPE, ZA showed beneficial effects by limiting the expression of pro-inflammatory and angiogenic molecules as well as the activity of the small GTP proteins RAS and RhoA (77). The first human study of ZA in MPE was recently published but its inconclusive results as to the treatment effect of ZA could be due to study design limitations (78). Another multicenter observational study comparing patients receiving the drug or not was terminated prematurely due to reduced patient recruitment (ClinicalTrials.gov Identifier: NCT00099541) (37). On the other hand, a recent study showed that a small molecule inhibitor, namely deltarasin, targets the membrane localization of KRAS, thus inhibiting oncogenic RAS signaling, and suppresses the in vitro and in vivo proliferation of human tumor cells that are dependent on oncogenic KRAS (79). This drug and other small molecule inhibitors may provide a novel opportunity to suppress oncogenic RAS signaling in experimental MPE. Inhibition of KRAS may be a more effective therapy for MPE.
The search for tumor transcriptional programs triggering MPE formation also identified nuclear factor (NF)-κB activation as an important component of the ability of lung adenocarcinoma cells to induce MPE development. NF-κB serves as a key signaling pathway linking inflammation with cancer (80,81). NF-κB activity in lung adenocarcinoma cells was central to their ability to cause MPE formation, as disruption of NF-κB signaling in these cells resulted in decreased tumor burden and reduced volume of MPE (43,82). NF-κB promotes secretion of tumor-elaborated TNF participating in an autocrine loop to sustain NF-κB activity (46). CCL2 expression in adenocarcinoma cells was also found to depend on NF-κB activation (47). These studies rendered NF-κB a novel therapeutic target. To this end, a recent study employed bortezomib, a proteasome inhibitor, to disrupt NF-κB activation in lung adenocarcinoma cells and reported beneficial effects against experimental MPE (83).
The EML4 (echinoderm microtubule-associated protein-like 4)-ALK (anaplastic lymphoma kinase) fusion oncogene, which is generated from a small inversion within human chromosome 2p leading to the expression of a chimeric tyrosine kinase, can drive the growth of some NSCLC (84). Crizotinib is a targeted drug that blocks EML4-ALK translocation-induced signaling (85) and is more effective than standard chemotherapy in patients with NSCLC showing this abnormality. Crizotinib is generally well tolerated and could be rapidly triaged in patients with MPE. Other specific abnormalities, such as mutations in the ROS-1 and RET genes are being studied and may lead to specific treatments for human MPE in the future.
Inhibition of tumor cell inflammatory signaling
It is clear that pleural malignant cells initiate an inflammatory crosstalk with host cells (pleural macrophages, lymphocytes, pleural mesothelial cells and endothelial cells) as well as with bone-marrow and lymphatic system-accrued cells (mononuclear cells, neutrophils, lymphocytes) which results in a wide accumulation of inflammatory and vasoactive mediators in the pleural space. These tumor- and host-originated inflammatory signals increase the vascular permeability leading to MPE formation. We and others have discovered different tumor or host derived chemokines and factors which play a significant role in MPE development (37,44,46). For example, host-derived IL-5 and tumor-derived TNF promoted experimental MPE and preclinical evidence supported the efficacy of IL-5 and TNF blockade against MPE formation (44,46).
Another important chemokine that was discovered is CCL2. Tumor-derived CCL2 can induce accumulation of myeloid cells to the pleural space and is necessary and sufficient for mouse MPE formation. Genetic ablation of CCL2 expression impaired several key aspects of MPE development including pleural mononuclear cell recruitment, new vessel formation and vascular leakage (47). Newly developed monoclonal antibodies directed against murine CCL2 and its murine ortholog CCL12 had identical effects with genetic ablation of CCL2 expression (86). Intriguingly, CCL2 and CCL12 showed redundancy; blockade of either or both of them had identical inhibitory effects on mononuclear cells and MPE development. Using a novel mouse model of human lung adenocarcinoma-induced MPE, the authors identify CCL2 blockade as a potential therapeutic approach for human MPE.
In addition, SPP1 was found to play an important role in MPE formation by promoting cancer cell survival and by regulating tumor-associated angiogenesis and inflammation, both central to the pathogenesis of MPE (48). SPP1 of host origin elicited macrophage recruitment into the pleural cavity and increased tumor angiogenesis, whereas tumor-derived SPP1 curtailed cancer cell apoptosis in vivo. SPP1 directly promotes vascular hyperpermeability. In addition, SPP1 of tumor and host origin differentially affected the expression of pro-inflammatory and angiogenic mediators in the tumor microenvironment. These results suggest that SPP1 of tumor and host origin impacts distinct aspects of MPE pathobiology to synergistically promote pleural fluid formation and pleural tumor progression. Other investigators have also concluded that tumor-derived SPP1is significant in MPE formation (87). One of the current goals in MPE therapy is to identify the most important immune players in MPE and block the crosstalk between tumor and host-immune cells. These chemokines and molecular agents discussed may present attractive targets for therapeutic interventions for patients with MPE.
Immunomodulation against MPE
Host immune cells have been identified as playing a prominent role in the pathobiology of MPE. These cells, which can either be present or recruited into the pleural space, are yet another potential target for MPE therapies. The most significant immune cell population present in pleural tumors and associated with MPE are macrophages, which have been shown to secrete a number of critical factors for MPE formation such as IL-6, CCL2 or SPP1 (45,47,48). Macrophages have two distinct phenotypes: M1 macrophages appear to impair tumor progression whereas M2 macrophages promote tumor progression (88). Interestingly, macrophages can be modulated using specific biologic interventions such as biphosphonate treatment, as shown in recent studies which reported that ZA exerted beneficial effects against MPE in part by modulating macrophage function (77,89). Furthermore, the use of 5,6-Dimethylxanthenone-4-acetic-acid (DMXAA, Vadimezan) was shown to modulate the activity of tumor associated macrophages and thus dramatically augment the effect of immunotherapy (90). A similar dual phenotype was recently described in pleural neutrophils recruited to tumors: neutrophils, controlled by TGF-β, can be discriminated into N1 (antitumor) or N2 (pro-tumor) polarizations (91). Myeloid derived suppressor cells, recruited to the pleural space by tumor-elaborated CCL2 and host-derived IL-5, are also important for pleural tumor progression via inhibition of effector T-cell function and can be effectively eliminated by conventional chemotherapy treatments (44,47,92). Host-derived IL-5 further attracts eosinophils to the pleural space (44). Lymphocytes can also impact pleural carcinomatosis as different lymphocyte populations exert different tumorigenic functions: CD4+ and CD8+ T-cells elicit antitumor effects in the pleural space, in contrast to regulatory T-cells which function in a tumor promoting way (93). Recent data from our laboratory also attribute a critical role to mast cells for MPE development (94). Mast cells are chemoattracted to the pleural space by tumor-elaborated CCL2 and trigger vasoactive effects essential for MPE formation. Interestingly, the cKIT inhibitor imatinib mesylate, which targets mast cells, was effective against experimental MPE.
The identification of the involvement of immune and inflammatory mechanisms towards an MPE promoting phenotype provides a rationale for the investigation of the potential application of immunomodulatory therapeutic approaches against MPE. Cancer immunotherapy, the notion of stimulating and enhancing the innate responses of the immune system against cancer, represents a promising cancer treatment approach. Interferons were of the first molecules studied in this context against MPE. Even though the first studies with interferon (IFN) administration were unsuccessful (95,96), more recent studies revealed promising results. Specifically, it was shown that after intrapleural administration of IFN alpha-2b in patients with MPE, no fluid recurrence was observed (97). In addition, a phase I clinical trial of repeated intrapleural doses of adenoviral vector expressing IFN-beta in patients with malignant pleural mesothelioma and MPE showed promising results (98). There is also an ongoing trial evaluating this approach of adenoviral IFN-beta gene transfer in pleural malignancies (ClinicalTrials.gov Identifier: NCT00299962). Although the exact mechanism of action of intrapleural IFN is not well understood, its immunomodulatory properties are likely to play a prominent role as IFN-alpha and IFN-beta are known to stimulate T cells, natural killer cells and macrophages (96,99). Intrapleural administration of IL-2 has also been addressed in phase I clinical studies with encouraging results which warranted further clinical investigation (100,101). A phase I trial is currently evaluating the effects of EMD 521873 (Selectikine), an IL-2/anti-DNA fusion protein designed to enrich IL-2 in tumor tissue, in patients with NSCLC and MPE (ClinicalTrials.gov Identifier: NCT00879866). Another immunotherapeutic approach is to attempt to provoke a broad immune response using nonspecific immunostimulatory agents like the inactivated bacterial super antigen (OK-432). Specifically, it has been shown that combined pleural treatment with OK-432 and IL-2 in patients with MPE decreased effusion volume in the majority of the patients (102). A phase II study, where patients with MPE were treated intrapleurally with OK-432 followed by standard chemotherapy regimen, reported improved survival time (103). Of a different context, a recent study reported on the significant enhancement of tumor immunotherapy by the combining blockade of CCL2 signaling with specific monoclonal antibodies, as a significant reduction in tumor volume was observed (104). These data support the clinical evaluation of immunotherapeutic agents as novel treatment of MPEs.
The role of interleukins in the pathobiology of MPE has been further elucidated in experimental mouse model studies. IL-6 was found to favor VEGF-mediated MPE formation through activation of Stat-3 (45). Host-derived IL-5 was present in mouse and human effusions and, importantly, ablation of host IL-5, either by the use of IL-5 devoid mice or by exogenous antibody treatment, produced a MPE resistant phenotype (44). Furthermore, a synergistic effect of IL-12 and IL-15 was described, which exerted antitumor activity and had beneficial effects on experimental MPE (105). A number of studies have described the presence of additional interleukins including IL-10 and IL-7 in pleural effusions; however their functional role in the phenomenon remains to be elucidated (106-108). Overall, it is becoming clear that the above findings can have significant therapeutic implications in the future.
Future perspectives
Pleural malignancies constitute a significant medical burden and merit coordinated translational research efforts to develop new and effective therapeutic modalities. The latest staging system for NSCLC upstaged the presence of MPE from T4 to M1a descriptor (109), highlighting the recognition of the complexity and severity of malignant pleural involvement in cancer care.
Major progresses have been achieved towards the elucidation of the pathobiology of MPE formation. Dissection of the biologic events and tumor properties favoring MPE development can yield additional therapeutic targets. For example, the identification of transcriptional networks essential for the tumor cells’ ability to cause MPE, like KRAS and NF-κB signaling, has intensified the research efforts towards unveiling the signaling cascades at play and discovering additional blocking agents of the above signaling pathways. Significant advances in basic and translational research are still needed to impact on clinical outcome of patients with MPE.
Acknowledgements
The authors are supported by European Research Council 2010 Starting Independent Investigator Grant #260524 (to GTS), by a Hellenic Thoracic Society 2012 Research Award (to ADG and GTS) and by a State Scholarship Foundation-IKY 2014 Scholarship (to MS).
Disclosure: The authors declare no conflict of interest.
References
- Henschke CI, Yankelevitz DF, Davis SD. Pleural diseases: multimodality imaging and clinical management. Curr Probl Diagn Radiol 1991;20:155-81. [PubMed]
- Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii32-40. [PubMed]
- Davies HE, Lee YC. Management of malignant pleural effusions: questions that need answers. Curr Opin Pulm Med 2013;19:374-9. [PubMed]
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001;18:402-19. [PubMed]
- Lee YC, Light RW. Management of malignant pleural effusions. Respirology 2004;9:148-56. [PubMed]
- Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc 2008;83:235-50. [PubMed]
- Heffner JE. Diagnosis and management of malignant pleural effusions. Respirology 2008;13:5-20. [PubMed]
- Khaleeq G, Musani AI. Emerging paradigms in the management of malignant pleural effusions. Respir Med 2008;102:939-48. [PubMed]
- Gompelmann D, Eberhardt R, Herth FJ. Advanced malignant lung disease: what the specialist can offer. Respiration 2011;82:111-23. [PubMed]
- Nam HS. Malignant pleural effusion: medical approaches for diagnosis and management. Tuberc Respir Dis (Seoul) 2014;76:211-7. [PubMed]
- Lee YC, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries: survey of pulmonologists. Chest 2003;124:2229-38. [PubMed]
- Burrows CM, Mathews WC, Colt HG. Predicting survival in patients with recurrent symptomatic malignant pleural effusions: an assessment of the prognostic values of physiologic, morphologic, and quality of life measures of extent of disease. Chest 2000;117:73-8. [PubMed]
- Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of pleurodesis failure: analysis of primary data. Chest 2000;117:87-95. [PubMed]
- Tan C, Sedrakyan A, Browne J, et al. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg 2006;29:829-38. [PubMed]
- Shaw P, Agarwal R. Pleurodesis for malignant pleural effusions. Cochrane Database Syst Rev 2004;CD002916. [PubMed]
- Walker-Renard PB, Vaughan LM, Sahn SA. Chemical pleurodesis for malignant pleural effusions. Ann Intern Med 1994;120:56-64. [PubMed]
- Paschoalini MS, Vargas FS, Marchi E, et al. Prospective randomized trial of silver nitrate vs talc slurry in pleurodesis for symptomatic malignant pleural effusions. Chest 2005;128:684-9. [PubMed]
- Mohsen TA, Zeid AA, Meshref M, et al. Local iodine pleurodesis versus thoracoscopic talc insufflation in recurrent malignant pleural effusion: a prospective randomized control trial. Eur J Cardiothorac Surg 2011;40:282-6. [PubMed]
- Burgers JA, Kunst PW, Koolen MG, et al. Pleural drainage and pleurodesis: implementation of guidelines in four hospitals. Eur Respir J 2008;32:1321-7. [PubMed]
- Dresler CM, Olak J, Herndon JE 2nd, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005;127:909-15. [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [PubMed]
- Fysh ET, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012;142:394-400. [PubMed]
- Putnam JB Jr, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer 1999;86:1992-9. [PubMed]
- Pollak JS, Burdge CM, Rosenblatt M, et al. Treatment of malignant pleural effusions with tunneled long-term drainage catheters. J Vasc Interv Radiol 2001;12:201-8. [PubMed]
- Tremblay A, Mason C, Michaud G. Use of tunnelled catheters for malignant pleural effusions in patients fit for pleurodesis. Eur Respir J 2007;30:759-62. [PubMed]
- Wrightson JM, Fysh E, Maskell NA, et al. Risk reduction in pleural procedures: sonography, simulation and supervision. Curr Opin Pulm Med 2010;16:340-50. [PubMed]
- Janes SM, Rahman NM, Davies RJ, et al. Catheter-tract metastases associated with chronic indwelling pleural catheters. Chest 2007;131:1232-4. [PubMed]
- Myers R, Michaud G. Tunneled pleural catheters: an update for 2013. Clin Chest Med 2013;34:73-80. [PubMed]
- Stathopoulos GT. Translational advances in pleural malignancies. Respirology 2011;16:53-63. [PubMed]
- Haas AR, Sterman DH. Novel intrapleural therapies for malignant diseases. Respiration 2012;83:277-92. [PubMed]
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [PubMed]
- Fysh ET, Tan SK, Read CA, et al. Pleurodesis outcome in malignant pleural mesothelioma. Thorax 2013;68:594-6. [PubMed]
- Han HS, Eom DW, Kim JH, et al. EGFR mutation status in primary lung adenocarcinomas and corresponding metastatic lesions: discordance in pleural metastases. Clin Lung Cancer 2011;12:380-6. [PubMed]
- Zocchi L. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J 2002;20:1545-58. [PubMed]
- Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997;10:1907-13. [PubMed]
- Meyer PC. Metastatic carcinoma of the pleura. Thorax 1966;21:437-43. [PubMed]
- Stathopoulos GT, Kalomenidis I. Malignant pleural effusion: tumor-host interactions unleashed. Am J Respir Crit Care Med 2012;186:487-92. [PubMed]
- Yano S, Shinohara H, Herbst RS, et al. Production of experimental malignant pleural effusions is dependent on invasion of the pleura and expression of vascular endothelial growth factor/vascular permeability factor by human lung cancer cells. Am J Pathol 2000;157:1893-903. [PubMed]
- Moschos C, Psallidas I, Kollintza A, et al. The angiopoietin/Tie2 axis mediates malignant pleural effusion formation. Neoplasia 2009;11:298-304. [PubMed]
- Nasreen N, Mohammed KA, Brown S, et al. Talc mediates angiostasis in malignant pleural effusions via endostatin induction. Eur Respir J 2007;29:761-9. [PubMed]
- Fang F, Chen P, Wu X, et al. Therapeutic effects of recombinant human endostatin adenovirus in a mouse model of malignant pleural effusion. J Cancer Res Clin Oncol 2009;135:1149-57. [PubMed]
- Gary Lee YC, Melkerneker D, Thompson PJ, et al. Transforming growth factor beta induces vascular endothelial growth factor elaboration from pleural mesothelial cells in vivo and in vitro. Am J Respir Crit Care Med 2002;165:88-94. [PubMed]
- Stathopoulos GT, Zhu Z, Everhart MB, et al. Nuclear factor-kappaB affects tumor progression in a mouse model of malignant pleural effusion. Am J Respir Cell Mol Biol 2006;34:142-50. [PubMed]
- Stathopoulos GT, Sherrill TP, Karabela SP, et al. Host-derived interleukin-5 promotes adenocarcinoma-induced malignant pleural effusion. Am J Respir Crit Care Med 2010;182:1273-81. [PubMed]
- Yeh HH, Lai WW, Chen HH, et al. Autocrine IL-6-induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene 2006;25:4300-9. [PubMed]
- Stathopoulos GT, Kollintza A, Moschos C, et al. Tumor necrosis factor-alpha promotes malignant pleural effusion. Cancer Res 2007;67:9825-34. [PubMed]
- Stathopoulos GT, Psallidas I, Moustaki A, et al. A central role for tumor-derived monocyte chemoattractant protein-1 in malignant pleural effusion. J Natl Cancer Inst 2008;100:1464-76. [PubMed]
- Psallidas I, Stathopoulos GT, Maniatis NA, et al. Secreted phosphoprotein-1 directly provokes vascular leakage to foster malignant pleural effusion. Oncogene 2013;32:528-35. [PubMed]
- Hsu IL, Su WC, Yan JJ, et al. Angiogenetic biomarkers in non-small cell lung cancer with malignant pleural effusion: correlations with patient survival and pleural effusion control. Lung Cancer 2009;65:371-6. [PubMed]
- Froudarakis ME. Pleural effusion in lung cancer: more questions than answers. Respiration 2012;83:367-76. [PubMed]
- Cheng D, Rodriguez RM, Perkett EA, et al. Vascular endothelial growth factor in pleural fluid. Chest 1999;116:760-5. [PubMed]
- Matsuyama W, Hashiguchi T, Mizoguchi A, et al. Serum levels of vascular endothelial growth factor dependent on the stage progression of lung cancer. Chest 2000;118:948-51. [PubMed]
- Zebrowski BK, Yano S, Liu W, et al. Vascular endothelial growth factor levels and induction of permeability in malignant pleural effusions. Clin Cancer Res 1999;5:3364-8. [PubMed]
- Zhou WB, Bai M, Jin Y. Diagnostic value of vascular endothelial growth factor and endostatin in malignant pleural effusions. Int J Tuberc Lung Dis 2009;13:381-6. [PubMed]
- Yano S, Herbst RS, Shinohara H, et al. Treatment for malignant pleural effusion of human lung adenocarcinoma by inhibition of vascular endothelial growth factor receptor tyrosine kinase phosphorylation. Clin Cancer Res 2000;6:957-65. [PubMed]
- Matsumori Y, Yano S, Goto H, et al. ZD6474, an inhibitor of vascular endothelial growth factor receptor tyrosine kinase, inhibits growth of experimental lung metastasis and production of malignant pleural effusions in a non-small cell lung cancer model. Oncol Res 2006;16:15-26. [PubMed]
- Pichelmayer O, Gruenberger B, Zielinski C, et al. Bevacizumab is active in malignant effusion. Ann Oncol 2006;17:1853. [PubMed]
- Wan X, Shen N, Mendoza A, et al. CCI-779 inhibits rhabdomyosarcoma xenograft growth by an antiangiogenic mechanism linked to the targeting of mTOR/Hif-1alpha/VEGF signaling. Neoplasia 2006;8:394-401. [PubMed]
- Vazakidou ME, Magkouta S, Moschos C, et al. Mammalian target of rapamycin (mTOR) inhibition does not prevent lung adenocarcinoma-induced malignant pleural effusion. Respirology 2014;19:290-2. [PubMed]
- Horn L, Sandler AB. Angiogenesis in the treatment of non-small cell lung cancer. Proc Am Thorac Soc 2009;6:206-17. [PubMed]
- Massarelli E, Onn A, Marom EM, et al. Vandetanib and indwelling pleural catheter for non-small-cell lung cancer with recurrent malignant pleural effusion. Clin Lung Cancer 2014;15:379-86. [PubMed]
- Tsigkos S, Koutsilieris M, Papapetropoulos A. Angiopoietins in angiogenesis and beyond. Expert Opin Investig Drugs 2003;12:933-41. [PubMed]
- Tait CR, Jones PF. Angiopoietins in tumours: the angiogenic switch. J Pathol 2004;204:1-10. [PubMed]
- Roviezzo F, Tsigkos S, Kotanidou A, et al. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther 2005;314:738-44. [PubMed]
- Lemieux C, Maliba R, Favier J, et al. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood 2005;105:1523-30. [PubMed]
- Venneri MA, De Palma M, Ponzoni M, et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood 2007;109:5276-85. [PubMed]
- Kalomenidis I, Kollintza A, Sigala I, et al. Angiopoietin-2 levels are elevated in exudative pleural effusions. Chest 2006;129:1259-66. [PubMed]
- Fang SC, Zhang HT, Hu HD, et al. Effect of Endostar combined with angiopoietin-2 inhibitor on malignant pleural effusion in mice. Med Oncol 2015;32:410. [PubMed]
- Zou J, Bella AE, Chen Z, et al. Frequency of EGFR mutations in lung adenocarcinoma with malignant pleural effusion: Implication of cancer biological behaviour regulated by EGFR mutation. J Int Med Res 2014;42:1110-7. [PubMed]
- Smits AJ, Kummer JA, Hinrichs JW, et al. EGFR and KRAS mutations in lung carcinomas in the Dutch population: increased EGFR mutation frequency in malignant pleural effusion of lung adenocarcinoma. Cell Oncol (Dordr) 2012;35:189-96. [PubMed]
- Shamblin CJ, Tanner NT, Sanchez RS, et al. EGFR mutations in malignant pleural effusions from lung cancer. Curr Respir Care Rep 2013;2:79-87.
- Pao W, Miller VA. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directions. J Clin Oncol 2005;23:2556-68. [PubMed]
- Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169-81. [PubMed]
- Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol 2012;13:e23-31. [PubMed]
- Belalcazar A, Azana D, Perez CA, et al. Targeting the Met pathway in lung cancer. Expert Rev Anticancer Ther 2012;12:519-28. [PubMed]
- Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol 2010;28:357-60. [PubMed]
- Stathopoulos GT, Moschos C, Loutrari H, et al. Zoledronic acid is effective against experimental malignant pleural effusion. Am J Respir Crit Care Med 2008;178:50-9. [PubMed]
- Clive AO, Hooper CE, Edey AJ, et al. A randomised controlled trial of intravenous zoledronic acid in malignant pleural disease: a proof of principle pilot study. PLoS One 2015;10:e0118569. [PubMed]
- Zimmermann G, Papke B, Ismail S, et al. Small molecule inhibition of the KRAS-PDEdelta interaction impairs oncogenic KRAS signalling. Nature 2013;497:638-42. [PubMed]
- Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004;431:461-6. [PubMed]
- Stathopoulos GT, Sherrill TP, Cheng DS, et al. Epithelial NF-kappaB activation promotes urethane-induced lung carcinogenesis. Proc Natl Acad Sci U S A 2007;104:18514-9. [PubMed]
- Stathopoulos GT, Sherrill TP, Han W, et al. Use of bioluminescent imaging to investigate the role of nuclear factor-kappaBeta in experimental non-small cell lung cancer metastasis. Clin Exp Metastasis 2008;25:43-51. [PubMed]
- Psallidas I, Karabela SP, Moschos C, et al. Specific effects of bortezomib against experimental malignant pleural effusion: a preclinical study. Mol Cancer 2010;9:56. [PubMed]
- Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010;363:1734-9. [PubMed]
- Ou SH, Bartlett CH, Mino-Kenudson M, et al. Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: a success story to usher in the second decade of molecular targeted therapy in oncology. Oncologist 2012;17:1351-75. [PubMed]
- Marazioti A, Kairi CA, Spella M, et al. Beneficial impact of CCL2 and CCL12 neutralization on experimental malignant pleural effusion. PLoS One 2013;8:e71207. [PubMed]
- Cui R, Takahashi F, Ohashi R, et al. Osteopontin is involved in the formation of malignant pleural effusion in lung cancer. Lung Cancer 2009;63:368-74. [PubMed]
- Hegmans JP, Veltman JD, Lambers ME, et al. Consolidative dendritic cell-based immunotherapy elicits cytotoxicity against malignant mesothelioma. Am J Respir Crit Care Med 2010;181:1383-90. [PubMed]
- Veltman JD, Lambers ME, van Nimwegen M, et al. Zoledronic acid impairs myeloid differentiation to tumour-associated macrophages in mesothelioma. Br J Cancer 2010;103:629-41. [PubMed]
- Fridlender ZG, Jassar A, Mishalian I, et al. Using macrophage activation to augment immunotherapy of established tumours. Br J Cancer 2013;108:1288-97. [PubMed]
- Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell 2009;16:183-94. [PubMed]
- Suzuki E, Kapoor V, Jassar AS, et al. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 2005;11:6713-21. [PubMed]
- Carroll RG, Carpenito C, Shan X, et al. Distinct effects of IL-18 on the engraftment and function of human effector CD8 T cells and regulatory T cells. PLoS One 2008;3:e3289. [PubMed]
- Giannou AD, Marazioti A, Spella M, et al. Mast cells mediate malignant pleural effusion formation. J Clin Invest 2015;125:2317-34. [PubMed]
- Yanagawa H, Haku T, Hiramatsu K, et al. Intrapleural instillation of interferon gamma in patients with malignant pleurisy due to lung cancer. Cancer Immunol Immunother 1997;45:93-9. [PubMed]
- Gebbia N, Mannino R, Di Dino A, et al. Intracavitary treatment of malignant pleural and peritoneal effusions in cancer patients. Anticancer Res 1994;14:739-45. [PubMed]
- Wilkins HE 3rd, Connolly MM, Grays P, et al. Recombinant interferon alpha-2b in the management of malignant pleural effusions. Chest 1997;111:1597-9. [PubMed]
- Sterman DH, Recio A, Haas AR, et al. A phase I trial of repeated intrapleural adenoviral-mediated interferon-beta gene transfer for mesothelioma and metastatic pleural effusions. Mol Ther 2010;18:852-60. [PubMed]
- Rambaldi A, Introna M, Colotta F, et al. Intraperitoneal administration of interferon beta in ovarian cancer patients. Cancer 1985;56:294-301. [PubMed]
- Astoul P, Viallat JR, Laurent JC, et al. Intrapleural recombinant IL-2 in passive immunotherapy for malignant pleural effusion. Chest 1993;103:209-13. [PubMed]
- Goey SH, Eggermont AM, Punt CJ, et al. Intrapleural administration of interleukin 2 in pleural mesothelioma: a phase I-II study. Br J Cancer 1995;72:1283-8. [PubMed]
- Yamaguchi Y, Miyahara E, Ohshita A, et al. Locoregional immunotherapy of malignant effusion from colorectal cancer using the streptococcal preparation OK-432 plus interleukin-2: induction of autologous tumor-reactive CD4+ Th1 killer lymphocytes. Br J Cancer 2003;89:1876-84. [PubMed]
- Ikehara M, Oshita F, Suzuki R, et al. Phase II study of OK-432 intrapleural administration followed by systemic cisplatin and gemcitabine for non-small cell lung cancer with pleuritis carcinomatosa. J Exp Ther Oncol 2004;4:79-83. [PubMed]
- Fridlender ZG, Buchlis G, Kapoor V, et al. CCL2 blockade augments cancer immunotherapy. Cancer Res 2010;70:109-18. [PubMed]
- Kimura K, Nishimura H, Matsuzaki T, et al. Synergistic effect of interleukin-15 and interleukin-12 on antitumor activity in a murine malignant pleurisy model. Cancer Immunol Immunother 2000;49:71-7. [PubMed]
- Yanagawa H, Takeuchi E, Suzuki Y, et al. Presence and potent immunosuppressive role of interleukin-10 in malignant pleural effusion due to lung cancer. Cancer Lett 1999;136:27-32. [PubMed]
- Chen YM, Yang WK, Whang-Peng J, et al. Elevation of interleukin-10 levels in malignant pleural effusion. Chest 1996;110:433-6. [PubMed]
- Klimatsidas M, Anastasiadis K, Foroulis C, et al. Elevated levels of anti inflammatory IL-10 and pro inflammatory IL-17 in malignant pleural effusions. J Cardiothorac Surg 2012;7:104. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]


