CT-guided percutaneous implantation of 125I particles in treatment of early lung cancer
Introduction
Currently, the 5-year survival of lung cancer is only about 15.6% (1). Early diagnosis and treatment are crucial in improving the long-term prognosis of lung cancer. The most widely used treatment approach for early lung cancer is surgical therapy; however, some patients do not receive surgical therapy clinically because of poor cardio-pulmonary function, physical weakness, advanced age or personal unwillingness. In previous studies, the high efficacy of the implantation of radioactive particles has been observed in the treatment of middle-to-late stage lung cancer. In a study by Yu et al. (2), the progression-free survival (PFS) was significantly prolonged and the local control rate (LCR) was significantly increased in the patients with stage III lung cancer who received 125I combined with chemotherapy. In another study by Han et al. (3), 39 patients with unresectable advanced lung cancer were given thoracoscopic implantation of 125I radioactive particles, and the response rate was 95.2% and 82.8% for postoperative atelectasis and pain, respectively. The survival rates were 97.4%, 89.7%, 56.4% and 46.2% at 6, 12, 18 and over 24 months after the procedure, respectively. Thus, the aim of this study was to analyze the efficacy of 125I radioactive particles for early lung cancer treatment.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2666).
Methods
General data
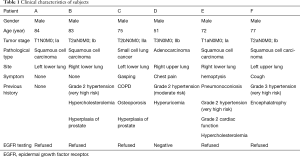
Six male patients with early lung cancer who were admitted to our hospital between June 2012 and April 2015 were included. Their age ranged from 51 to 84 years (73.67±12.0 years on average). Four of them had squamous cell carcinoma,1 had adenocarcinoma, and 1 had small cell lung cancer. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Fujian Medical University (approval No. 2012-002). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all the patients.
The clinical characteristics are listed in Table 1.
Full table
Inclusion criteria
Definition and diagnosis of early lung cancer was based on the criteria in the staging system of lung cancer established by the American Thoracic Society in 2013 (4), where stage I and II were defined as early lung cancer.
The inclusion criteria of patients were the following: (I) pathologically confirmed lung cancer; (II) stage I and II lung cancer in accordance with TNM staging system; (III) inability or personal unwillingness to receive surgical therapy due to advanced age, multiple underlying diseases and/or physical weakness; (IV) simultaneously estimated survival exceeding 6 months; (V) no history of any previous treatment for the cancer at enrollment time (e.g., external beam radiotherapy, chemotherapy, etc.); (VI) no other types of treatment applied for the cancer after implantation of the particle; (VII) positive for indication of implantation of 125I radioactive particles.
The exclusion criteria of patients was the following: (I) coagulation disorder; (II) inability to receive percutaneous pulmonary puncture due to serious cardiac, hepatic and renal insufficiency, or serious pulmonary infection; (III) active hemorrhage, necrosis or ulcer at the site of tumor; (IV) contraindication to implantation of radioactive particle.
Device and instrument
Hispeed NX/i dual-slice spiral CT (GE) and Model 6,711 125I sealed seed source (HTA. Co. Ltd.) were utilized, and the particle was loaded into the particle bin. 125I radioactive particles were 4.5 mm in length and 0.8 mm in diameter. The appearance was a cylindrical titanium alloy package, the penetration distance in tissue was 1.7 cm, the average energy of 125I particle was 27.4–35.5 keV, the activity of single particle was 0.7–0.8 mci, and the half-life was 59.43 d. Qualified particles were transported to the hospital in accordance with a type A package through a leak hunting test and activity measurement before release. An 18G radioactive particle source implant needle and turntable type implant gun (Japanese Medicine Co. Ltd.) were the devices used for radioactive particle source implantation. The implant needle had 1 scale on the surface and could be connected with an implant apparatus. The radioactive particle treatment planning system was provided by Zhuhai Hokai Medical Equipment Co., Ltd.
Preoperative preparation
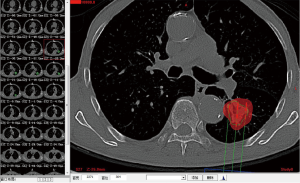
Complete blood cell count, full panel of coagulation function, and ECG were performed routinely for all the patients prior to the operation. A pulmonary plain + contrast-enhanced CT scan was performed to observe the tumor and its blood supply carefully. Three-dimensional treatment planning system (TPS) software was used to calculate the therapeutic dose, amount, spacing and distribution of seeds in the therapeutic target area and adjacent tissues. The amount of particle required for implantation was determined by the following formula: (tumor length + tumor width + tumor height)/3(cm)×5/activity per particle (mCi). The preoperative planning and dose volume histogram (DVH) were completed (Figures 1,2). Prior to the operation, the patients were informed of the course of operation, signed the informed consent form, and instructed to do breathing exercises.
Implantation method
The surgeons wore lead clothes with collars. The scanning position was determined in accordance with the CT image. The patient was placed in supine, prone and lateral positions, with the head advanced at first. The CT scan was then performed; the optimal entry point and direction of path were verified, the depth of entry was drawn, and a mark was carefully made. Routine disinfection and draping were performed. The patient was instructed to hold breath after local infiltration anesthesia with 2% lidocaine commenced. The insertion of the puncture needle stopped at the depth measured. The patient was then asked to breathe. The CT scan could be performed at any time during the procedure as to adjust the depth and direction of puncture. As much as possible, the center of the tumor was used as the entry point. The number of particles was calculated based on the spacing of 0.5–1.0 cm. The particles were implanted according to the plan. Distribution of particles was observed through scanning, the area with less distribution was replanted, and pulmonary CT scan was performed immediately after the procedure as to observe the presence of pneumothorax and hemothorax. The heart rate and blood oxygen saturation were closely observed during the procedure. While hemostatics were applied for the prevention of hemorrhage, the patients were told to lie on a bed to rest, wear lead clothes, and avoid radiation injury to other people around them after the procedure.
Postoperative follow-up
In order to monitor the remission of clinical symptoms, the patients were followed up 1, 3, 6, 12, 18, 24, 30 and 36 months after procedure. The pulmonary CT, and evaluation examination of tumor markers and other organs were repeated.
Evaluation criteria on response
The response was evaluated based on the change observed in the longest diameters of target lesions measured in radiological examination before and after treatment, in accordance with the response evaluation criteria in solid tumors (RECIST) version 1.1 (5), which delineates criteria for complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD). CR was defined as the disappearance of all the target lesions, reduction of the short diameter of all the pathological lymph nodes (including target nodule and non-target nodule) to <10 mm, and completely normal tumor markers. PR was defined as the reduction of the sum of diameters of target lesions by at least 30% from baseline. SD was defined as the reduction of the sum of diameters of target lesions not reaching PR, or an increase not reaching PD (i.e., somewhere in between PR and PD), using the minimal sum of diameters as reference in the study. PD was defined as the relative increase of the sum of diameters by at least 20% (using the baseline value as reference if the baseline measurement was the minimum) with an absolute increase of 5 mm (occurrence of one or more new lesions was also regarded as PD), using the minimal sum of the diameters of all the target lesions measured throughout the study as reference.
Observations
The side effects of radiotherapy, including nausea, vomiting, headache, dizziness, bone marrow suppression, and remission of clinical symptoms during follow-up were observed after particle implantation. At the same time, the clinical response was evaluated according to tumor markers which were primarily referred to as neuron-specific enolase (NSE), carcinoembryonic antigen (CEA), and cytokeratin 19 fragments (Cyfra 21-1). Tumor reduction and presence of recurrence or metastasis at follow-up visits were additionally evaluated. Occurrence of complications after particle implantation was observed, and response rate (RR), and disease control rate (DCR) were calculated according to the following formulae: RR = (CR + PR)/total number of cases, and DCR = (CR + PR + SD)/total number of cases.
Statistical method
Direct statistical method was used for statistical analysis of the efficacy and complications. SPSS19.0 software was utilized for plotting the change of tumor size.
Results
General data
(I) Reasons for non-surgical treatment in the 6 patients were the following: advanced age in one patient (patient A), advanced age and multiple underlying diseases in 4 patients (patient B, C, E and F), and personal unwillingness in one patient (patient D). (II) Time of enrollment was between June 2012 and November 2015, as shown in Figure 3. (III) In the pulmonary lesions treated with 125I radioactive particles, the longest tumor diameter was 6.04 cm, and the shortest diameter was 2.04 cm (3.79±1.37 cm on average). At each site, 20–55 particles were implanted, for a total of 226 particles. One patient received an implantation of 125I radioactive particles twice.
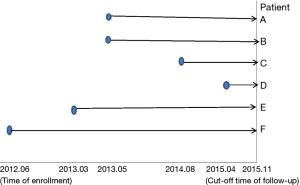
As can be seen from Figure 3, all 6 patients survived. The 5 patients who were followed up for 1 year had a 1-year survival rate of 5/5 (100%), and the 4 patients who were followed up for 2 years had a survival rate of 4/4 (100%). The longest time of follow-up after enrollment was 3 years and 5 months, and the patient still survived.
Particle implantation
The course of treatment went well. The implantation rate was 100%. One patient had low fever with a temperature of 38.1 °C one day after implantation. The temperature decreased to normal on day 3 after an administration of sulperazone for anti-infection therapy. No hemoptysis, chest distress or chest pain occurred. Two patients had moderate pneumothorax after implantation and were given closed drainage of the thoracic cavity. The bedside chest X-ray repeated 5 days later showed complete absorption of gas. No complications seriously affecting lifestyle occurred in the other patients. No particle misplacement, intrathoracic massive hemorrhage, or other serious complications occurred in any of the patients.
Follow-up
The patients were followed up 1, 3, 6, 12, 18, 24, 30 and 36 months after procedure. The follow-up is displayed in Table 2.

Full table
Follow-up results
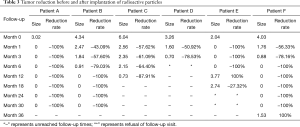
In accordance with the longest diameter of the tumors measured in radiological examination, using the longest diameter to represent tumor size (cm), reductions of tumors during follow-up are presented in Table 3.
Full table
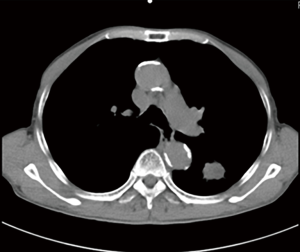
As illustrated in Table 3, tumors were significantly reduced in all 6 patients after the implantation of radioactive particles. A typical case can be seen in Figures 4,5.
Using the longest diameter of tumor as a longitudinal coordinate (cm), and follow-up time as a horizontal coordinate, change of tumor size is displayed in Figure 6.
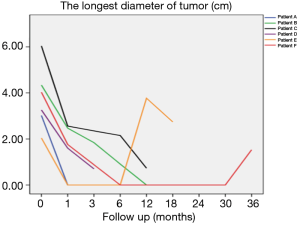
Figure 6 also shows that the tumors significantly decreased in all 6 patients after implantation of radioactive particle, which is consistent with the findings in Table 3. The most obvious decrease can be seen in the 6th month after procedure.
No nausea, vomiting, headache or dizziness occurred in any of the patients after implantation. The completed blood cell test showed no bone marrow suppression. The clinical symptoms were significantly relieved in all the patients. During follow-up, recurrence of primary lesion and left lung metastasis occurred in patient E in the 12th month after procedure, recurrence of primary lesion occurred in patient F in the 36th after procedure, while no recurrence or metastasis occurred in the other patients. No patient had distant metastasis during follow-up. No radiation pneumonia, radiation esophagitis, esophago-tracheal fistula, particle drop-off, migration or other serious complications occurred. The results of repeated tumor marker tests in the 6 patients during follow-up are visible in Table 4.

Full table
Evaluation of response on tumor
Pulmonary CT was repeated 1, 3, 6, 12, 18 and 24 months after procedure, so as to evaluate local tumor control in the 6 patients after treatment. The results can be seen in Figure 7.
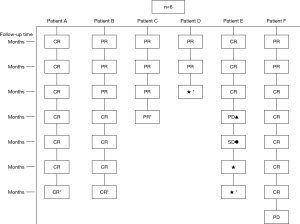
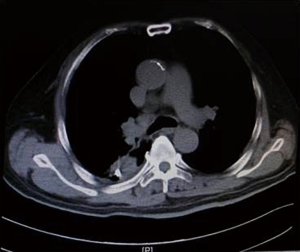
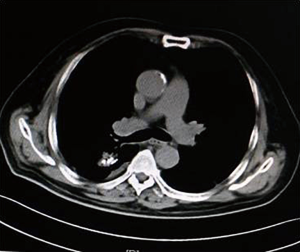
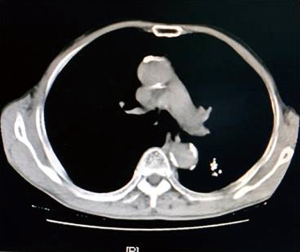
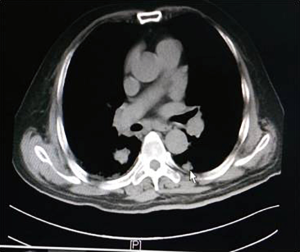
As shown in Figure 7 and Figure 3, there were 2 CRs and 4 PRs after follow-up for 1 month, indicating a response rate (RR) of 6/6, and a disease control rate (DCR) of 6/6; six patients were followed up for 6 months, including one patient who refused follow-up visit (still alive),while the other 5 patients were followed up regularly, including 3 CRs and 2 PRs; five patients were followed up for 12 months, including 3 CRs, 1 PR and 1 PD, indicating an RR of 4/5, a DCR of 4/5, and a progression rate of 1/5; four patients were followed up for 18 months, including 3 CRs and 1 SD, indicating a RR of 3/4 and a DCR of 4/4; four patients were followed up for 24 months, including 1 patient who refused a follow-up visit (still alive), while the other 3 patients were still followed up regularly; there were still 4 patients who were followed up for 30 months, including 1 patient who refused follow-up visit (alive) and 3 patients who were followed up on a regular basis; one patient was followed up for 36 months and had a recurrence of primary lesion, with the response being evaluated as PD. In addition, Figure 7 shows that patient E achieved significant response after implantation of 125I particles, and the response was evaluated as CR 1 month after implantation (Figures 8,9); however, the primary lesion recurred and was enlarged with left lung metastasis in month 12 (Figures 10,11), whereupon the response was evaluated as PD. The patient was given CT-guided implantation of 125I radioactive particles again for treatment of the recurrent lesion. The repeated pulmonary CT after re-implantation showed significant reduction of tumor (reduction rate 27.32%), as shown in Figure 12, and the response was subsequently re-evaluated as SD.
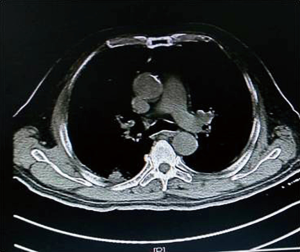
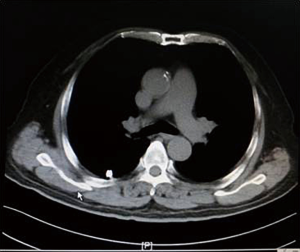
Discussion
Clinically, stage I and II lung cancer are defined as early lung cancers according to the TNM staging system. Treatment for early lung cancer includes surgery as the first choice, then radiochemotherapy and interventional therapy. However, there is still a small portion of patients who do not receive surgery due to multiple underlying diseases, physical weakness, advanced age, or personal unwillingness. In this study, 4/6 patients were unable to receive surgery for advanced age and multiple underlying diseases, while 1 patient was unable to receive surgery due to advanced age, and 1 patient did not opt for surgery for personal reasons. The question then arises concerning which therapy should be used to maximally prolong the survival extent of these kinds of patients.
In recent years, with the increasing popularity of interventional technology for respiratory diseases, a series of novel therapeutic approaches have been made available for such patients. These interventional technologies include implantation of radioactive particles, percutaneous thermal ablation (thermal radiofrequency, microwave ablation) and percutaneous cryotherapy. More and more studies have found the implantation of radioactive particles in the treatment of middle-to-late stage lung cancer to be efficacious. This evidence begs the further question of whether the implantation of 125I radioactive particles could also achieve ideal efficacy in treatment of unresectable early lung cancer. This article is intended to preliminarily analyze the therapeutic value of radioactive particle in early lung cancer.
Treatment of malignant tumors with implantation of radioactive particles has a history of almost 100 years. In 1914, Pasteau and Degrais innovated the first use of radioactive particles for treatment of malignant tumors by applying the implantation of radioactive particle radium in the treatment of prostate cancer. As an example of a borderline science between oncological surgery and oncological radiology, this therapy involved implanting particles around or inside tumors and emitting irradiation therapy using the beam released from the particles (6). Much later that century, in 1999, Imamura et al. (7) implanted a high dose of radionuclide 192Ir through a percutaneous puncture of a lung cancer patient who could not tolerate surgical therapy due to poor pulmonary function. The tumor significantly shrank after the procedure and in the 2 years of follow-up, no enlargement, radiation pneumonia, esophago-tracheal fistula, or other radiation damage occurred. The study demonstrated, for the first time, a number of important observations relating to the implantation of a high dose of radionuclide: an overall safe and effective treatment of tissues, few adverse reactions in the treatment of the lung cancer, improved therapeutic dose in the target area, reduction of the dose received by surrounding normal tissues, reduction of radiation damage to surrounding normal tissues, and the surpassing of the relative shortage of fractionated short-term irradiation in traditional external radiotherapy.
In the present study, 125I radioactive particles were implanted for treatment of early lung cancer, with a 125I particle penetration distance of 1.7 cm into the tissue to greatly reduced the occurrence of radiation damage to surrounding normal tissues. No resulting side effects of radiotherapy, including nausea, vomiting, dizziness and bone marrow suppression, occurred after the implantation in any of the patients in this study. All of the above side effects were significantly reduced compared to the effects observed in traditional external radiotherapy. Moreover, in traditional external radiotherapy, the prescription dose is only 40–70 Gy in treatment of lung cancer, which cannot reach the radical dose needed for treatment of tumors. The recommended prescription dose of 125I was 140–160 Gy (8), which significantly increased the irradiation dose for tumors, reduced the chance of tumor recurrence and metastasis, and improved survival rate—benefits which on the whole further confirm Imamura’s assertions (7).
TPS software was used to calculate the therapeutic dose, amount, spacing and distribution of seeds in the therapeutic target area and the adjacent tissues in this study. The successful implantation rate was 100%, which indicates that the use of TPS software to formulate a plan allowed a more homogeneous and rational distribution of particles while improving the success rate of implantation. Compared with traditional external radiotherapy, inter-tissue implantation of radioactive particle also had two other major advantages (9). First, the continuous irradiation of γ-ray released by radioactive particle 125I significantly reduced the chance of tumor re-proliferation. Low-dose continuous irradiation inhibited mitosis of tumor cells that had stagnated in the G2 phase. The G2 phase was sensitive to radiation, which was conducive to killing tumors, and thus local therapy lasted for a long time and had remarkably high efficiency. Second, the location of the therapy was accurate and coincided with the shape of tumor. As a result, the implantation of radioactive particle played a critical role in the treatment of malignant tumors.
Other relevant studies have shown a unique advantage of implantation of radioactive particles in the treatment of lung cancer. In a study by Lu et al. (10), 15 patients with obstructive pneumonia caused by central type lung cancer were given a bronchoscopic implantation of 125I radioactive particles, and followed up in months 2, 6, 12, 18 and 24 after implantation. The median survival was 15.6 months, and the one-year lung recruitment rate was 80.0%, which significantly improved the quality of life. To compare, in our study’s response evaluation from regular follow-up of patients who received the CT-guided implantation of radioactive 125I, it was found that the tumors had significantly shrunk in all 6 patients. Typical cases are illustrated in Figures 4 and 5, and as can be seen in Figure 6, the most obvious reduction of tumor was seen in the 6th month of follow-up. In the 5 patients who were followed up, there were 3 CRs, indicating an RR of 5/5 (100%) and a DCR of 5/5 (100%). This shows that the implantation of radioactive particle achieved the best response within 6 months in the treatment of early lung cancer. In addition, as Figure 3 shows, 5 patients were followed up 1 year after implantation and had a survival rate of 5/5 (100%), 4 patients were followed up 2 years after implantation and had a survival rate of 4/4 (100%), and the longest time after enrollment was up to 3 years and 5 months; at the time of writing, this patient is still alive. The observations above demonstrate the significant efficacy of the implantation of 125I radioactive particles in treatment of early lung cancer.
This conclusion was also validated in the study by Li et al. (11), where 24 patients with unresectable T1–3N0M0 non-small cell lung cancer received CT-guided implantation of 125I radioactive particles and were followed up for 31.5 months on average after implantation. The local control rate (LCR) was 78.6%, 1-, 2- and 3-year survival rates were 95.8%, 78% and 55% respectively, and the median survival was 38 months. Conversely, in a study by Zhang et al. (12), it was shown that the 1-, 2- and 3-year survival rates were 53%, 44% and 24% respectively in the patients who received traditional external radiotherapy for early non-small cell lung cancer, which indicated a significantly better response of implantation of radioactive particles in the treatment of early lung cancer than traditional external radiotherapy.
The median survival could not be calculated temporarily due to the short follow-up time in this study. CT-guided implantation of radioactive particle was used, and no massive hemothorax, particle misplacement, or other serious complications occurred in any of the patients during the implantation. This shows that the implantation of the 125I radioactive particles not only had a significant response in the treatment of unresectable early lung cancer, but also provided the best treatment for patients, significantly improved their survival rate and quality of life, and consequently brought patients a new overall hope for the future. Under the guidance of CT, the tissues around the tumor could be clearly detected as to avoid damage to normal tissue and the surrounding blood supply. Thus, the operation was safe, and the therapeutic dose, the number of particles implanted, and the spacing and distribution of seeds in the therapeutic target area and its adjacent tissues were made clear in accordance with the TPS planning system which improved the accuracy of particle implantation for the treatment of the target lesions.
In this study, no radiation pneumonia, radiation esophagitis, esophago-tracheal fistula, particle drop-off, migration, or other serious complications occurred in any of the 6 patients during follow-up, which shows that the inter-tissue implantation of radioactive particle was safe, reliable, and had few complications. In the study by Chen et al. (13), 23 patients with stage I non-small cell lung cancer and unsatisfactory cardio-pulmonary function were given 125I radioactive particles for treatment and followed up for 11 months. The repeated chest CT scan showed no 125I particle migration or local recurrence in any of the patients. No obvious decreasing trend was seen in the repeated pulmonary function tests on a regular basis. Similarly, Zhang et al. (14) analyzed 34 patients with locally advanced non-small cell lung cancer who had failure of the first chemotherapy. All the 34 patients received CT-guided implantation of radioactive particle and were followed up for 32 months on average. No radiation pneumonia, radiation esophagitis, esophago-tracheal fistula or other serious life-threatening complications occurred in any of the patients during follow-up, which further supports our viewpoint concerning the safety, efficacy, and lack of complications involved in the CT-guided implantation of radioactive particle in treatment of early lung cancer.
In our study, as can be seen in Figures 7-11, in the 5 patients who were followed up regularly 1 year after implantation of radioactive particle, only 1 patient had pulmonary metastasis and obvious enlargement of the primary lesion. The response for this patient was evaluated as PD, which might be related with the half-life of 59.43 days for an 125I radioactive source. Only 1.6% of the original energy was left 1 year (6 half-lives) later, and the lethality was significantly reduced, thus possibly explaining the recurrence. Might a routine re-implantation of 125I radioactive particle then be required one year after implantation to prolong the time to recurrence, improve therapeutic effect and prolong the survival of patients who received this type of treatment? A study by Meng et al. (15) found a positive effect of the implantation of radioactive particles in treatment of recurrent head and neck cancer after external radiotherapy. It is worth asking then if a re-implantation of radioactive particles could achieve efficacy in the recurrent lesions in patients with recurrence after inter-tissue brachytherapy. In our study, the patients whose response was evaluated as PD 1 year after implantation again received CT-guided implantation of 125I radioactive particles for treatment of recurrent lesions. The subsequent repeated pulmonary CT 6 months after procedure showed re-reduction of tumor (Figure 12), with a reduction rate of 27.32%. This indicates that the implantation of radioactive particles could be repeated in treatment of malignant tumors; however, the response needs to be further observed in a larger sample size with a more prolonged follow-up time in order to more firmly establish this claim. Additionally, as the killing capability of rays varied in tumors of different tissue type, the question arises of whether the γ-rays released by the radioactive particles would still be effective against the recurrent tumor after it had mutated, or if an increase in dose would be required. As there have been no studies exploring this issue, it should be further analyzed after relevant samples have been collected.
Although CT-guided implantation of 125I radioactive particle had significant efficacy in treatment of early lung cancer, these positive results would not have been achieved if the implantation had not been rationally implemented. The key for percutaneous particle implantation in treatment of lung cancer is the appropriate dose and distribution of particles. Compactness of particle in the peripheral areas and sparseness in the center are critical in achieving homogenous dose distribution. On the other hand, inaccurate particle spacing or deflection of the implant needle can lead to heterogeneous dose distribution. Thus, prior to implantation, the implantation regimen should be devised by a computed stereotaxic treatment planning system (TPS) in order to ensure, to the best of ability, that distribution of radioactive particles is homogeneous, that there is equal spacing between particles, and that the radioactive source is on the same line and mutually parallel.
In addition, it should be remembered that because the implantation of radioactive particles is an invasive procedure, certain complications such as, pneumothorax, hemoptysis, postoperative infection, particle drop-off, and in particular, pneumothorax and hemoptysis, may appear during and after the procedure. In this study, moderate pneumothorax occurred in 2 patients during the procedure, and fever occurred in 1/6 of the patients 1 day after the procedure. In a study by Ming et al. (16), where 15 patients received implantation of radioactive particle for treatment of lung cancer, 3 patients had hemoptysis, 2 patients had pneumothorax, and 1 patient had particle drop-off during the procedure. Multiple adjustments of the depth and direction of the puncture during the procedure can aggravate the occurrence of pneumothorax, and so precise location of the puncture and breathing exercises of patients were particularly important. Meanwhile, pleura needed to be anaesthetized sufficiently during the procedure as to reduce cough, sudden and strenuous exertion, operation time, and the occurrence of pneumothorax. The patients were told to lie on a bed to rest and were given oxygen inhalation in case of a small amount of pneumothorax, which could be absorbed spontaneously. When a large amount of pneumothorax occurred, closed drainage of the thoracic cavity was performed to recruit the compressed lungs as early as possible. Contrast-enhanced pulmonary CT scan was required prior to procedure, as to identify the blood supply and vessels traveling in the tumor and surrounding tissues, and to avoid major vessels during the procedure. At the same time, to reduce the occurrence of hemoptysis, attention also needed to be paid to platelet coagulation function and the use of anticoagulants. A study by Shen et al. (17) found that a thoracic implantation of radioactive particles could also induce acute radiation pneumonia and pulmonary fibrosis. Similarly, Zhang et al. (14) reported that a small area of pulmonary fibrosis occurred around the particle seeding area, and therefore, accurate quantification of the particles implanted to avoid over-implantation is important for preventing the occurrence of this adverse effect. However, no pulmonary fibrosis or acute radiation pneumonia occurred in the present study, which could be attributed to the small number of cases; thus, in a study with a larger sample size, the potential emergence of these effects should be kept in mind for investigation.
Other adverse effects of implantation include loss, migration and drop-off of radioactive particles. In 1991, Steinfeld et al. (18), first reported a pulmonary embolism following pulmonary migration after implantation of 125I for early prostate cancer. In the study by Ming et al. (16), where 15 patients received implantation of radioactive particles for treatment of lung cancer, 1 patient had particle drop-off during the procedure. No loss, migration and drop-off of radioactive particles occurred in our study, which also might be related with the small number of cases involved.
Although implantation of radioactive particles achieved good response in treatment of a variety of malignant tumors, it also had certain disadvantages. First, it was shown in the study by Zhang et al. that implantation metastasis could be caused during implantation of radioactive particles (19). Zhang et al. observed that a new lesion was found in the implant needle path during postoperative follow-up in 1 of 29 patients who received implantation of 125I radioactive particles, which could not be excluded from metastasis. Consequently, the following needed to be attended to during the procedure: (I) selecting a site going through the least normal tissue for puncture; (II) using a new puncture needle for different lesions and different patients, as to ensure one-to-one use; (III) placing the needle core inside the needle guard when removing the puncture needle, then pulling both out together. The study also discovered that it was hard for the radioactive particle to achieve ideal therapeutic effect in those tumors that were progressing rapidly (20), because the effective treatment time of radioactive particles was correlated with the doubling time of tumor cells. If the doubling time was short, the dose after the effective treatment time, i.e., the invalid dose, would be increased correspondingly.
Some shortcomings were present in this study: (I) the half-life of 125I particle was 60 d, and so, due to the decreased radiation dose after numerous half-lives, the possibility of local recurrence needs to be further confirmed through an increase of sample size and intensified follow-up time; (II) no control group was designed, and so it is impossible to be sure whether implantation of radioactive particles is superior to other therapeutic regimens; (III) the sample size was inadequate; (IV) currently, partial patients still have not completely reached the follow-up time; therefore, the long-term efficacy of implantation of 125I radioactive particles cannot yet be statistically analyzed.
Conclusions
The preliminary clinical observation showed implantation of 125I radioactive particles was a safe and effective minimally-invasive method in the treatment of early lung cancer.
Acknowledgments
Funding: This work was funded by Science and Technology Major Project of Fujian Province, China (2017YZ001-2).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2666
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2666
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2666). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Fujian Medical University (approval No. 2012-002). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reyes ME, Schabath MB. Optimal lung cancer screening intervals following a negative low-dose computed tomography result. J Thorac Dis 2019;11:S1916-8. [Crossref] [PubMed]
- Yu X, Li J, Zhong X, et al. Combination of Iodine-125 brachytherapy and chemotherapy for locally recurrent stage III non-small cell lung cancer after concurrent chemoradiotherapy. BMC Cancer 2015;15:656. [Crossref] [PubMed]
- Han Z, Zhang Z, Ren H, et al. Clinical significance of 125I radioactive seeds implanting by video-assisted thoracoscopic surgery in treatment of advanced lung cancer. China Journal of Endoscopy 2008;14:900-5.
- Detterbeck FC, Postmus PE, Tanoue LT. The stage classification of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e191S-210S.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guide line (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Khan FM. The Physics of Radiation Therapy. 6th ed. Philadelphia: Lippincoll Williams & Wilkins, 2012:786.
- Imamura F, Chatani M, Nakayama T, et al. Percutaneousbrachy therapy for small-sized non-small cell lung cancer. Lung Cancer 1999;24:169-74. [Crossref] [PubMed]
- Raben A, Mychalczak B. Brachytherapy for non-small cell lung cancer and selected neoplasms of the chest. Chest 1997;112:276S-86S. [Crossref] [PubMed]
- Hu X, Qiu C, Lv D, et al.The study and evaluation of curing metaphase or terminal lung cancer by embedding 125I under CT guidance. Cancer Research and Clinic 2006;(7):509-511.
- Lu M, Pu D, Zhang W, et al. Trans-bronchoscopy with implantation of 125I radioactive seeds in patients with pulmonary atelectasis induced by lung cancer. Oncol Lett 2015;10:216-22. [Crossref] [PubMed]
- Li J, Yu M, Xiao Y, et al. Computed tomography fluoroscopy-guided percutaneous 125I seed implantation for safe, effective and real-time monitoring radiotherapy of inoperable stage T1-3N0M0 non-small-cell lung cancer. Mol Clin Oncol 2013;1:1019-24. [Crossref] [PubMed]
- Zhang L, Wang L, Zhang H, et al. Treatment result of radiotherapy for medically inoperable stage I/II non-small cell lung cancer. Chin J Radiat Oncol 2008;17:101-5.
- Chen A, Galloway M, Landreneau R, et al. Intraoperative 125I brachytherapy for high-risk stage I non-small cell lung carcinoma. Int J Radiat Oncol Biol Phys 1999;44:1057-63. [Crossref] [PubMed]
- Zhang T, Lu M, Peng S, et al. CT-guided implantation of radioactive 125I seed in advanced non-small-celllung cancer after failure of first-line chemotherapy. J Cancer Res Clin Oncol 2014;140:1383-90. [Crossref] [PubMed]
- Meng N, Jiang YL, Wang JJ, et al. Permanent implantation of iodine-125 seeds as a salvage therapy for recurrent head and neck carcinoma after radiotherapy. Cancer Invest 2012;30:236-42. [Crossref] [PubMed]
- Ming H, Zou C, Zhou Q, et al. 125I seed implantation combined with chemotherapy in treatment of non-small cell lung cancer in advanced stage. Chin J Inter Rad (Electronic Edition) 2014;2:42-6.
- Shen W, Wang L. Radioactive Damage. Beijing: China Medical Science Press, 2001:95-103.
- Steinfeld AD, Donahue BR, Plaine L. Pulmonary embolization of iodine-125 seeds following prostate implantation. Urology 1991;37:149-50. [Crossref] [PubMed]
- Zhang S, Zheng Y, Yu P, et al. The combined treatment of CT-guided percutaneous 125I seed implantation and chemotherapy for non-small-cell lung cancer. J Cancer Res Clin Oncol 2011;137:1813-22. [Crossref] [PubMed]
- Liu X, Bao Y, Lin Y. Clinical study on chemotherapy combined radioactive seed interstitial brachytherapy for locally advanced non-small cell lung cancer. Chin J Clin Oncol 2013.992-6.
(English Language Editor: J. Gray)