
The five-year outcome of the transcatheter aortic valve replacement in the partner 2A study in patients with intermediate surgical risk—what is clear and what it is unclear
The 5-year results of the PARTNER 2A study of the transcatheter aortic valve replacement (TAVR) in patients with intermediate surgical risk were recently published in the New England Journal of Medicine (1). At its first publication in the same journal, in July 2017 (2), the study was accepted by the scientific community as a confirmation that transcatheter technique can also be applied to patients with intermediate-risk aortic stenosis. Precisely in light of the results of the PANTER 2A study and the Pivotal trial (3) (who used the self-expanding (SE) valve in the same type of patients), the ESC guidelines published in the same year considered in Class 1 level of evidence B the indication for surgical replacement or percutaneous implantation of the aortic valve in patients with STS score ≥4 (or with other comorbidities not including risk scores such as fragility, the presence of porcelain aorta or previous chest radiation therapy) with evaluation by the heart team and preferring the percutaneous procedure in elderly patients and when the procedure is feasible for transfemoral access (4,5).
Treating intermediate-risk patients, however, has rekindled the discussion especially regarding the durability of the percutaneous valve (6). For this reason, the publication of the results at 5 years has provided new insights on the percutaneous treatment of aortic stenosis. The Partner 2A study included a total of 2,032 patients with symptomatic severe aortic stenosis, with intermediate surgical risk (STS score ≥4%) in 57 centers in the United States and Canada (2). Patients were further stratified according to the possible access route (trans-femoral or transthoracic) based on imaging studies, particularly computed tomography (CT), and were then randomly assigned (in a ratio of 1:1) to the TAVR group or the traditional surgery group (2).
Of the 2,032 patients in the study, 1,550 (76.3%) were eligible for transfemoral access and the remaining 482 (23.7%) were randomized to transthoracic access (1). The average age of the patients was 81.6 years and the average STS score was 5.8%. Also, in this trial as in the previous PARTNERS, women are well represented (45.5% of patients) compared to trials on ischemic heart disease and stents. Coronary revascularization using coronary artery bypass grafting (CABG) was performed simultaneously in 137 of 944 patients in the surgical group. Coronary revascularization by angioplasty (PCI) was performed in 39 of 994 patients among TAVR candidates.
Mortality and stroke
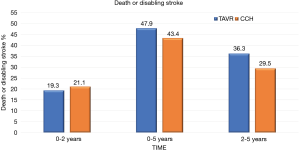
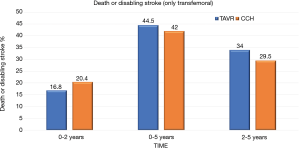
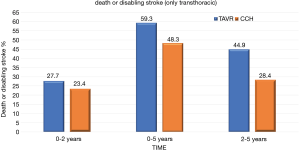
At 5 years, there was no significant evidence in the incidence of the composite endpoint of all-cause mortality or disabling stroke between the TAVR group and the surgical group (47.9% and 43.4% respectively; hazard ratio, 1.09; 95% CI, 0.95–1.25; P=0.21) (Figure 1). In the cohort of patients treated with transfemoral access, the incidence of all-cause mortality or disabling stroke was similar in the TAVR group and in the surgical group (44.5% and 42.0% respectively; risk ratio, 1.02; 95% CI, 0.87–1.20) (Figure 2). However, the incidence of death or disabling stroke was higher after TAVR compared to traditional surgery when the procedure was performed trans-thoracically (59.3% vs. 48.3%; hazard ratio, 1.32; 95% CI, 1.02–1.71) confirming once again that the superiority of the percutaneous procedure over the surgical one is valid only for the transfemoral procedures (Figure 3). The incidence of all-cause mortality in the TAVR group and the surgery group was 46.0% and 42.1%, respectively, in the overall population, 42.7% and 40.5% in the cohort of transfemoral access and 56.9% and 47.3% in the transthoracic access cohort.
Rehospitalization and reintervention
The 5-year data show that re-hospitalization occurred more frequently after TAVR than after surgery (33.3% vs. 25.2%; hazard ratio, 1.28; 95% CI, from 1, 07 to 1.53).
Aortic valve reoperation was a rare event in both groups but occurred more frequently among patients in the TAVR group than in the surgical group (3.2% vs. 0.8%; hazard ratio, 3.28; 95% CI, 1.32–8.13). The most frequent causes of reoperation are due to progressive valve stenosis (10 out of 21 cases) or significant residual aortic regurgitation (11 out of 21 cases). Most patients (18 out of 21) were treated with a TAVR in TAVR or by performing balloon catheter valvuloplasty. Endocarditis was the main cause of reoperation in patients in the surgical group (4 out of 6 cases), most of whom were treated with further surgery.
Echocardiographic parameters
Echocardiographic assessments performed at five years documented the presence of at least mild paravalvular aortic regurgitation in 33.3% of patients in the TAVR group and in 6.3% of patients in the surgical group.
Moderate-severe paravalvular regurgitation (PVR) occurred more frequently with TAVR than surgery during the 5-year follow-up period and was associated with an increased risk of death from any cause.
Precisely in light of the close correlation between the extent of regurgitation and mortality, significant engineering improvements have been made to the devices and implant techniques.
While the device used in the Partner 2A study, the SAPIEN XT, is no longer in clinical use, the subsequent evolution, the Sapien 3 valve (Edwards Lifesciences), and the SAPIEN 3 Ultra, currently in use, use an external fabric skirt that increases sealing with the annulus. These new valves are implanted by correctly assessing the size of the annulus with CT and are associated with moderate or severe PVR incidences significantly lower both post-procedurally and at 1 year compared to what observed with the previous generation of devices. So the results of the Partner 2A study remain obsolete and not are useful in clinical practice as the new device has been associated with moderate-severe PVR rates of less than 1% in low-risk patients (7), percentage significantly lower than the 6,5% observed with the Sapien XT valve used in PARTNER 2A.
The echocardiographic follow up of the study documented a good performance of both surgical and percutaneous valves: however, the area of the aortic valve is smaller in the surgical group, although the average gradient did not differ between the groups.
New York Heart Association (NYHA) class and re-hospitalization
Cardiac symptoms and quality of life have improved in both the TAVR and surgical arm, with prolonged benefits for 5 years.
In the five-year follow-up, both TAVR and surgery led to improvements in health status (both in the NYHA functional class and in the KCCQ-OS score).
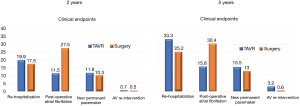
At five-year follow-up, TAVR documented greater risks of a procedure or valve-related rehospitalization and more aortic valve reoperations, but a lower risk of postoperative atrial fibrillation than surgery (Figure 4). These findings raised the question of which hospital should be selected for the re-admission after TAVR (8).
However, the incidence of death or incapacitating stroke was higher after TAVR compared to traditional surgery when the procedure was performed trans-thoracically (59.3% vs. 48.3%; hazard ratio, 1.32; 95% CI, 1.02–1.71) confirming once again that the superiority of the percutaneous procedure over the surgical one is valid only for the transfemoral procedures (9).
Discussion
Analyzing the follow-up data available after percutaneous or surgical aortic valve implantation, landmark analysis was carried out between 2 and 5 years which documented a not significant incidence of death from any cause or invalidating stroke (1).
As the authors themselves point out, however, these data cannot be extended to the entire intermediate-risk population both because the average age is still advanced (81 years) and because the device used in the original trial is not the one currently in use, as we already pointed out.
Gaps of Evidence of TAVR. Although TAVR was introduced 14 years ago, there are still many gaps of evidence (Table 1).
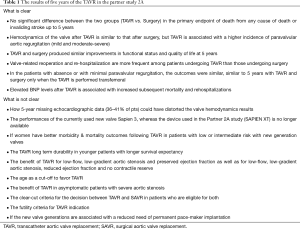
Full table
The benefit of TAVR in patients with low-flow, low-gradient aortic stenosis, and preserved ejection fraction remains largely unknown today. Future studies should clarify the clinical benefit of TAVR in this patient population.
The benefit of TAVR in asymptomatic patients with severe aortic stenosis and the use of left ventricular dysfunction biomarkers in this population remains to be determined. Finally, whereas in older patients the risk stratification is well defined (10).
Clear criteria for the indication to TAVR or surgery in low- intermediate-risk, when both are eligible, as well as dear futility criteria for TAVR need to be redefined. The use of dedicated medical APPs could be useful to help the decision-making process (11).
A key only partially solved issue is the valve durability after TAVR. Definitely, five-year data from Partner A study cannot be considered long-term durability data for either TAVR or traditional surgery.
Although echocardiographic data have not been fully reported in the 5-year Partner2A study, data from the literature indicate that in the long-term good durability of the TAVR for both SE and balloon-expandable (BE) valves, with the maintenance of a valve area and an average gradient adequate and superimposable to the post-procedural (12).
The long-term valves durability (>15 years) needs to be studied, especially in patients of 75-year-old, or younger. The ESC guidelines favor indications for TAVR in patients aged 75 and over. This cut-off, which is not based on studies, needs to be redefined on the basis of trials.
Current data demonstrated that the long-term transcatheter aortic valve function is excellent with 91% of patients remained free of structural valve degeneration (SVD) between 5- and 10-years post-implantation and the incidence of severe SVD was <1% (13).
It should be pointed out that in case of valve dysfunction after TAVR, Redo-TAVR is a relatively safe and effective option for selected patients with valve (14).
A recent study that compared device performance of a BE transcatheter heart valve (THV) versus a SE demonstrated similar clinical outcome with both valves.
Moderate or severe structural valve deterioration was uncommon but occurred more frequently with the BE valve (15).
However, long term follow-up from clinical trials including younger patients with longer survival expectancy are needed to definitely assess the TAVR long term durability.
Although in the Partner trial, moderate-severe PVR occurred more frequently with TAVR than surgery, data from clinical studies show that TAVR with new generation systems has shown a significant improvement in terms of reduction of paravalvular leakage and is associated with extremely good clinical results (7). The new devices are so powerful as to obtain excellent results even in the ascending phase of the learning curve (16,17).
The reduction of the paravalvular leaks also reduces the re-hospitalization and the need for reoperation and is associated with increased survival.
The future challenge is to have an ideal prosthetic valve available with minimal risk and discomfort to the implant, hemodynamics similar to that of a normal valve, which does not require anticoagulants, and which lasts for the entire life of the patient (18).
The phenomenon of prosthesis-patient mismatch is more frequent in patients treated with surgery, especially in women. In the case of smaller annulus, the phenomenon possible with the surgical aortic valve replacement (SAVR) does not occur with the TAVR.
A recent study reported the prognostic significance and potential clinical utility of natriuretic peptide levels measured after valve replacement (19). In this study, elevated BNP levels after TAVR were independently associated with increased subsequent mortality and rehospitalizations.
Finally, there are many data in the literature showing a better long-term outcome in women than men. The women with severe aortic stenosis are frequently more old, fragile with smaller femoral artery diameters, left ventricular hypertrophy, small LV dimensions, a greater prevalence of porcelain aorta, smaller aortic ring sizes and lower coronary ostium heights. On the other hand, they may have less cardiovascular comorbidity, less atherosclerotic disease, and better left ventricular systolic function. Although after TAVR women have more vascular complications and more frequent need for blood transfusion and a higher incidence of stroke, the long-term outcomes appear better than men (20). This gender gap seems to decrease and disappear in low-risk patients, where the one-year outcomes are similar in both genders.
Finally, in the PARTNER 2A the percentage of patients after TAVR with a new permanent pacemaker at 30 days was 8.5% (vs. 6.9% of surgery, P=0.17). However, a systematic analysis at a nationwide level found higher rates of permanent pace-maker implantation (PPI) than previously reported. Balloon Expandable technology was independently associated with lower incidence rates of PPI both at the acute and chronic phases than SE technology (21).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was a free submission to the journal. The article was sent for external peer review.
Provenance and Peer Review: This article was a free submission to the journal. The article was sent for external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1205). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Makkar RR, Thourani VH, Mack MJ, et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N Engl J Med 2020;382:799-809. [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [PubMed]
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis. N Engl J Med 2014;370:1790-8. [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [PubMed]
- Spaccarotella C, Mongiardo A, De Rosa S, et al. Transcatheter aortic valve implantation in patients at intermediate surgical risk. Int J Cardiol 2017;243:161-8. [PubMed]
- Polimeni A, Sorrentino S, De Rosa S, et al. Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients for the Treatment of Severe Aortic Stenosis. J Clin Med 2020;9:439. [PubMed]
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med 2019;380:1695-705. [PubMed]
- Spaccarotella C, Mongiardo A, Sorrentino S, et al. Which hospital should be selected for readmission after TAVR? Int J Cardiol 2019;293:107-8. [PubMed]
- Indolfi C, Bartorelli AL, Berti S, et al. Updated clinical indications for transcatheter aortic valve implantation in patients with severe aortic stenosis: expert opinion of the Italian Society of Cardiology and GISE. J Cardiovasc Med (Hagerstown) 2018;19:197-210. [PubMed]
- Pulignano G, Gulizia MM, Baldasseroni S, et al. ANMCO/SIC/SICI-GISE/SICCH Executive Summary of Consensus Document on Risk Stratification in elderly patients with aortic stenosis before surgery or transcatheter aortic valve replacement. Eur Heart J Suppl 2017;19:D354-69. [PubMed]
- Indolfi C, Sabatino J, De Rosa S, et al. Description and Validation of TAVIApp: A Novel Mobile Application for Support of Physicians in the Management of Aortic Stenosis-Management of Aortic Stenosis with TAVIApp. Biomed Res Int 2017;2017:9027597. [PubMed]
- Russo MJ, McCabe JM, Thourani VH, et al. Case Volume and Outcomes After TAVR With Balloon-Expandable Prostheses: Insights From TVT Registry. J Am Coll Cardiol 2019;73:427-40. [PubMed]
- Blackman DJ, Saraf S, MacCarthy PA, et al. Long-Term Durability of Transcatheter Aortic Valve Prostheses. J Am Coll Cardiol 2019;73:537-45. [PubMed]
- Landes U, Webb JG, De Backer O, et al. Repeat Transcatheter Aortic Valve Replacement for Transcatheter Prosthesis Dysfunction. J Am Coll Cardiol 2020;75:1882-93. [PubMed]
- Abdel-Wahab M, Landt M, Neumann FJ, et al. CHOICE Investigators. 5-Year Outcomes After TAVR With Balloon-Expandable Versus Self-Expanding Valves: Results From the CHOICE Randomized Clinical Trial. JACC Cardiovasc Interv 2020;13:1071-82. [PubMed]
- Solomonica A, Choudhury T, Bagur R. Newer-generation of Edwards transcatheter aortic valve systems: SAPIEN 3, Centera, and SAPIEN 3 Ultra. Expert Rev Med Devices 2019;16:81-7. [PubMed]
- Spaccarotella C, Mongiardo A, Curcio A, et al. Will transcatheter aortic valve implantation represent the choice treatment for all patients who need a biological valve? J Cardiovasc Med (Hagerstown) 2020;21:345-8. [PubMed]
- Otto CM. Informed Shared Decisions for Patients with Aortic Stenosis. N Engl J Med 2019;380:1769-70. [PubMed]
- O'Leary JM, Clavel MA, Chen S, et al. Association of Natriuretic Peptide Levels After Transcatheter Aortic Valve Replacement With Subsequent Clinical Outcomes. JAMA Cardiol 2020.e202614. [PubMed]
- Mihos CG, Klassen SL, Yucel E. Sex-Specific Considerations in Women with Aortic Stenosis and Outcomes After Transcatheter Aortic Valve Replacement. Curr Treat Options Cardiovasc Med 2018;20:52. [PubMed]
- Bisson A, Bodin A, Herbert J, et al. Pacemaker Implantation After Balloon- or Self-Expandable Transcatheter Aortic Valve Replacement in Patients With Aortic Stenosis. J Am Heart Assoc 2020;9:e015896. [PubMed]


