Quantitation and predictors of short-term mortality following extrapleural pneumonectomy, pleurectomy/decortication, and nonoperative management for malignant pleural mesothelioma
Introduction
A relatively uncommon but highly aggressive neoplasm, malignant pleural mesothelioma (MPM) poses major challenges to multidisciplinary management (1). While guidelines endorse the use of first-line cytotoxic chemotherapy and adjuvant radiation therapy in surgical patients, the routine use of chemotherapy and radiation therapy have both been questioned by phase III trials (2,3). Surgical therapy in the form of extrapleural pneumonectomy (EPP) or pleurectomy/decortication (P/D) is considered the cornerstone of therapy for operable patients (1), but it is also not supported by high-level evidence from randomized clinical trials (4).
However, extrapolation of the aforementioned trials to clinical practice remains difficult, largely owing to noteworthy limitations. The SAKK study of radiotherapy was underpowered (2,5), and the MS01 investigation utilized a regimen that is no longer the standard of care (3,6). Most strikingly, the MARS trial demonstrated an infeasibility to conduct a surgical randomized trial and documented a high perioperative mortality rate (12.5%) in the surgical (EPP) arm, which could have dampened the possible outcome advantages offered by surgery (7).
The chief lesson from MARS was that because resection is a morbid procedure, patients must be carefully selected pre-operatively in order to minimize post-operative morbidities and mortality, the presence of which can reduce the degree of surgical benefits. Careful patient selection and improvements in technical experience and postoperative care have led to reductions in mortality rates (8). For example, patient-related factors, including age (≥65 years), male sex, forced expiratory volume in 1 second (FEV1) (<60% of predicted), and lower preoperative hemoglobin level, convey a higher risk for perioperative mortality (9). The presence of patient comorbidities, such as peripheral vascular disease, cerebrovascular disease, congestive heart failure and benign lung disease, are also independently associated with poor perioperative outcomes. In addition to patient characteristics, several treatment-related factors, including the operation type (EPP) and surgical center volume (<5 procedures per year), have been shown to predict for increased perioperative mortality (10). While EPP carries 30-day mortality rates of 4–7% (2,11,12), the lung-sparing P/D procedure has been increasingly utilized in contemporary periods and has been demonstrated to have fewer complications and equivalent or superior survival as compared to EPP (11,13-18). However, although the MARS 2 trial (NCT02040272) continues to accrue, an analogous study to MARS has not been completed utilizing P/D; hence, the role of resection remains in flux, and both techniques are endorsed by the National Comprehensive Cancer Network (NCCN) (1).
In order to refine pre-operative patient selection, as well as to address the controversy regarding surgical approaches (EPP vs. P/D), it is imperative to better quantify and evaluate predictors of short-term (30- and 90-day) mortality with EPP versus P/D, as well as with nonoperative management. This investigation, therefore, aimed to address this knowledge gap by examining the large, contemporary National Cancer Database (NCDB). We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1779).
Methods
The NCDB is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society that consists of information regarding tumor characteristics, patient demographics, and patient survival for approximately 70% of the United States population (19). The American College of Surgeons and the CoC have not verified and are neither responsible for the analytic or statistical methodology employed nor the conclusions drawn from these data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). As all patient information in the NCDB is de-identified, this study was exempt from institutional review board evaluation. The NCDB Participant User File corresponding to mesothelioma [2004–2013] was utilized for this study.
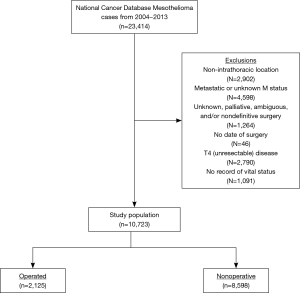
Methodological analysis of this study mirrored recent studies of other neoplasms, including testing the hypothesis that postoperative mortality was related to age (20,21). As a result, short-term (30- and 90-day postoperative) mortality was compared between EPP and P/D (previously reported NCDB codes identifying each surgical procedure) (18,22,23) using a priori age-based intervals (≤60, 61–65, 66–70, 71–75, 76–80, and ≥81 years). Because this comparison required technical resectability and proper documentation of surgical technique, subjects with T4 (unresectable) disease as well as those who underwent nondefinitive/palliative or ambiguous/unknown surgery were excluded. Of note, there was no exclusion based on nodal status, because (I) there are a multitude of data showing that well-selected cases can attain appropriate survival with low short-term mortality, and (II) resection is an option in NCCN guidelines for well-selected node-positive patients (1,4,24-26). Other exclusion criteria were lack of a coded vital status, primary mesothelioma location in a non-intrathoracic site, and/or metastatic (or unknown) disease.
Additionally, because is important to quantify the short-term mortality risk in the unresected population as well, a nonoperative cohort was also separately analyzed (having received chemotherapy, radiotherapy, chemoradiotherapy, or supportive care). For this cohort, 30- and 90-day mortality was defined from the time of initial diagnosis. No comparisons of mortality between the surgical and nonsurgical cohorts was made, owing to a multitude of uncontrolled retrospective selection biases and fundamental discrepancies in defining the 30- and 90-day windows.
Statistical analysis
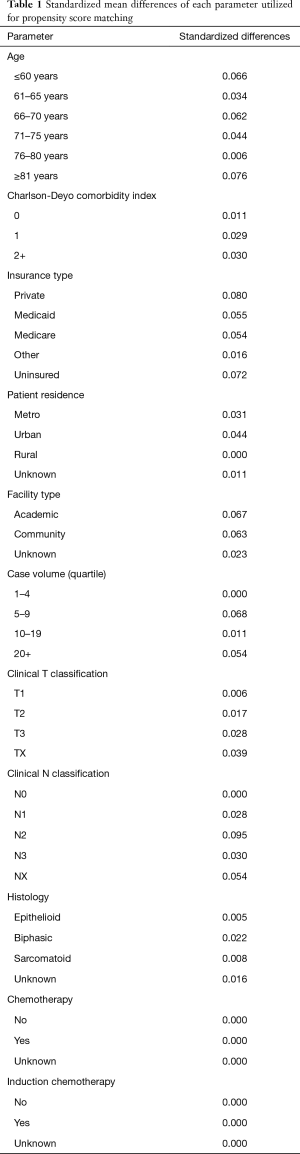
In accordance with the variables in NCDB files, information collected on each patient broadly included demographic, clinical, and treatment data. Statistical analysis was performed with STATA version 14 (College Station, TX, USA). Tests were two-sided, with a threshold of P<0.05 for statistical significance. First, clinical characteristics of the overall cohort were tabulated. Second, cumulative incidences of mortality by treatment paradigm were then tabulated and graphed for each age interval as well as the whole population. Propensity matching was utilized to better balance groups (27-29). Propensity scores were calculated using a multivariable logistic regression model, with the dependent variable being the particular treatment technique and the independent variables being those that were statistically significant for correlation with mortality on multivariable analysis. Patients were matched 1:1 without replacement to avoid potential bias from many-to-one matching. Standardized differences were assessed to ensure balance between each of the variables included in calculating the propensity score to the matched cohorts, with a value <0.1 signifying an inconsequential imbalance (Table 1) (30). Third, interaction testing was utilized to create forest plots aiming to evaluate the interaction between age and hazard ratio (HR) for mortality between treatment paradigms. Lastly, Cox multivariable analysis was performed to ascertain factors independently associated with 30- and 90-day mortality. We present the following article in accordance with the STROBE reporting checklist (31).
Full table
Results
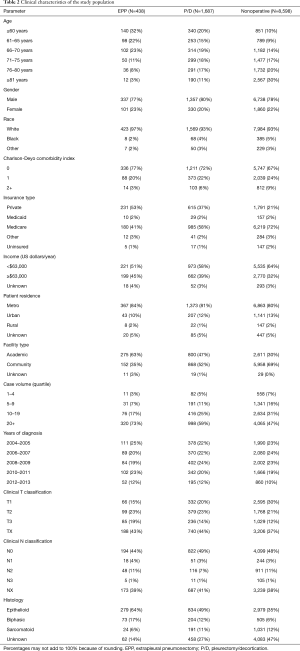
A patient selection diagram is illustrated in Figure 1. Overall, 10,723 patients met study criteria (Table 2). Of these, 2,125 (19.8%) received resection (n=438 EPP, n=1,687 P/D) and 8,598 (80.2%) underwent nonoperative management. Of note, a plurality of patients were node-negative and treated at facilities in the upper quartile of case volume (defined by the total number of cases over the NCDB study period). Chemotherapy was delivered to 303 (69.2%) subjects in the EPP group (n=122 induction chemotherapy), 928 (55.0%) in the P/D cohort (n=194 induction), and 3,656 (42.5%) of the nonoperative cases.
Full table
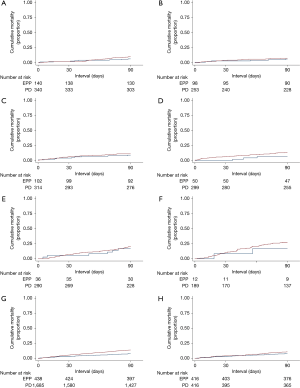
The 30-day mortality was 3.0% and 5.4% for EPP and P/D, respectively; corresponding 90-day mortality figures were 8.0% and 14.1% (Table 3). When aggregated, the overall unadjusted 30- and 90-day mortality rates for resected patients were 4.9% and 12.8%, respectively. Figure 2 displays cumulative incidences of short-term mortality by receipt of EPP versus P/D, displaying no differences within each age group (P=0.222, 0.647, 0.547, 0.155, 0.635, and 0.447, respectively, for ages ≤60, 61–65, 66–70, 71–75, 76–80, and ≥81 years). Although there was a statistical difference when evaluating all subjects (P=0.001), this difference did not persist following propensity matching (P=0.193).

Full table
Additionally, despite the lack of differences between EPP and P/D within each age-based subgroup, interaction testing was performed to further evaluate HRs for mortality between age-related (unadjusted) groups. Forest plots are shown in Figure 3, demonstrating that the effect size was not significantly different between the six age-based cohorts at 30 (P=0.958) or 90 (P=0.955) days.
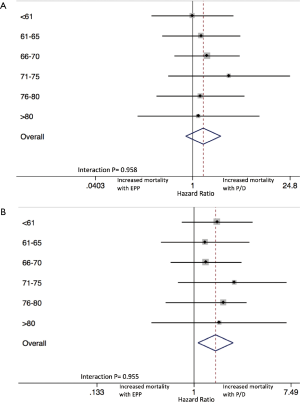
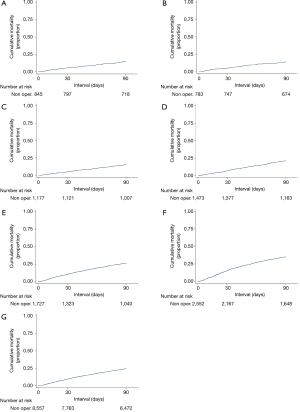
Figure 4 illustrates short-term mortality of the nonoperated cohort. For these high risk patients, the crude 30- and 90-day mortality was 9.9% and 24.6%, respectively (Table 3).
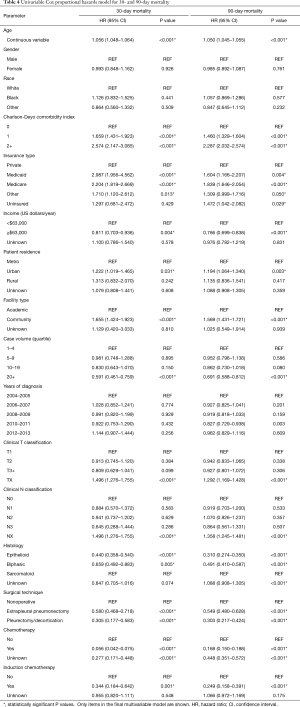
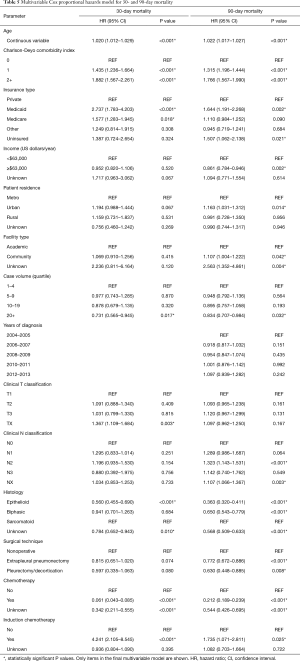
Following univariable evaluation (Table 4), multivariable Cox analysis was performed to assess factors predicting for 30- and 90-day mortality (Table 5). Variables associated with both outcomes included comorbidities, insurance, case volume, histology, and chemotherapy. Of note, surgical patients having received induction chemotherapy were associated with higher 30- and 90-day mortality (P=0.025), whereas treatment at high-volume centers was associated with less mortality (P=0.032). Age was also significantly associated with both 30- and 90-day mortality (P<0.001); each yearly incremental increase in age (at diagnosis) conferred a 2.0% increased risk of 30-day mortality and 2.2% increased risk of 90-day mortality.
Full table
Full table
Discussion
MPM management continues to be debated; the decision to resect, as well as the optimal technique thereof, remain controversial. A quantitative understanding of mortality risk (and predictors thereof) for several approaches is thus critical to better refine patient selection. The largest such study of its kind, this investigation of a large, contemporary national database quantitates age-associated 30- and 90-day mortality of EPP and P/D, which should be considered when potentially operable patients are counseled regarding the risks and benefits of resection.
The reader is strongly cautioned when interpreting these data, largely owing to confounding by operability status in NCDB studies, which results in well-selected surgical patients and the vast majority of nonoperative patients being unresectable/inoperable. As a result, if a potentially operable patient is weighing resection versus lack thereof, the clinician can obtain a quantifiable estimate of that patient’s estimated 30- and 90-day postoperative mortality based on age and the proposed surgical technique (Table 3). However, short-term mortality risks in potentially operable patients declining resection cannot be ascertained from this study because they are not synonymous with the nonoperative group herein.
These data also imply that nonoperative (largely unresectable and/or inoperable) patients have a high baseline mortality, a notion that has been under-studied from a quantitative perspective (Table 3). Given the high rate of short-term mortality in certain subsets (e.g., older patients with greater comorbidities), these data may question whether nonoperative candidates should receive aggressive therapies (i.e., systemic therapy and/or radiotherapy) near the end of life (32), a notion that has been promulgated from randomized trials (3) and retrospective data (29).
This investigation additionally sheds light into potentially modifiable factors of short-term mortality, such as therapy at high-volume facilities (33) and lack of induction chemotherapy (34). These should be integrated with a thorough pre-operative assessment if surgery is being planned. This assessment should comprise of several important factors not coded in the NCDB, including performance status, cardiopulmonary function, volume and location of disease, and mental/emotional tolerance of the proposed therapy. Additional factors such as age, nodal status, and histology can be added to create a “complete clinical picture” on which the decision to operate is made.
The mortality figures herein are roughly comparable to existing data (2,10-17). They are, however, considerably lower than the MARS data, which documented a 30-day mortality (11%) numerically similar to operated patients ≥81 years of age in this study (10.4%) (7). However, the median age of the MARS population was 62, which is difficult to extrapolate to a more “general” MPM population whose median age at diagnosis is nearly a decade older (1).
There are several noteworthy shortcomings of any retrospective NCDB study, in addition to the lack of important information not coded in the NCDB mentioned above (34,35). First, the goal of this investigation was not to evaluate overall mortality (examined elsewhere) (10,13-18); although examining short-term mortality attenuates many biases associated with assessment of overall mortality, lead-time and immortal time bias can never be eliminated. It also cannot be ascertained whether “short-term mortality” in this paper equated to “treatment-related mortality,” since specific complications and/or causes of mortality are not given in the NCDB. This likely explains the discrepancy between the lack of postoperative mortality differences between EPP and P/D herein, as compared to other studies showing fewer complications with P/D (10,12-17). Importantly, comparisons made between patients treated with surgery and nonoperative management is subject to significant clinical heterogeneity, including but not limited to differences in patient surgical fitness or tolerability. MPM conveys a poor short-term prognosis irrespective of surgical versus nonoperative management. Herein, we sought to corroborate that the short-term mortality conveyed by EPP or P/D was not associated with increases in baseline short-term mortality compared to nonoperative management. Additionally, a major limitation of including both induction and postoperative chemotherapy (cycles and agents of which are not given in the NCDB) is that every operated patient who received induction chemotherapy remained alive until resection; in other words, those who died following induction were likely a part of the nonoperative group. Next, it is acknowledged that the nonoperative subjects, who have known gender biases (36), were a notably heterogeneous population, comprising of those who were “fit” enough to tolerate chemotherapy (likely resulting in the significant association with 30- and 90-day mortality on Cox modeling) as well as those too frail to receive any oncologic therapy. These disparate patients were deliberately merged similar to an intention-to-treat analysis, where patients are analyzed together regardless of whether they were able to receive the intervention. It also allowed for a more “real-world” viewpoint of expected mortality rates (rather than artificial inflation if the supportive care subjects were removed, for instance). Furthermore, specific selection criteria influencing the surgical approach, including patient characteristics and surgeon’s preferences, are not made available via the NCDB and represents an additional limitation of this study. Lastly, although the NCDB includes data for 70% of the United States population, only CoC-accredited facilities contribute data; as such, these findings may not necessarily be representative of the entire United States population.
Conclusions
As MPM management remains controversial, a quantitative understanding of mortality risk (and predictors thereof) for several treatment approaches is thus critical to better refine patient selection. The largest such study of its kind, this investigation of a large, contemporary national database quantitates age-associated 30- and 90-day mortality of EPP and P/D, which should be considered when potentially operable patients are counseled regarding the risks and benefits of resection.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1779
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1779
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1779). CB Simone 2nd serves as an unpaid editorial board member of Journal of Thoracic Disease from Jan 2020- Dec 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). As all patient information in the NCDB is de-identified, this study was exempt from institutional review board evaluation. The NCDB Participant User File corresponding to mesothelioma [2004–2013] was utilized for this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Comprehensive Cancer Network. Malignant pleural mesothelioma (Version 1.2020). Available online: https://www.nccn.org/professionals/physician_gls/pdf/mpm.pdf
- Stahel RA, Riesterer O, Xyrafas A, et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): A randomised, international, multicentre phase 2 trial. Lancet Oncol 2015;16:1651-8. [Crossref] [PubMed]
- Muers MF, Stephens RJ, Fisher P, et al. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial. Lancet 2008;371:1685-94. [Crossref] [PubMed]
- Sugarbaker DJ, Richards WG, Bueno R. Extrapleural pneumonectomy in the treatment of epithelioid malignant pleural mesothelioma novel prognostic implications of combined N1 and N2 nodal involvement based on experience in 529 patients. Ann Surg 2014;260:577-80. [Crossref] [PubMed]
- Rimner A, Simone CB 2nd, Zauderer MG, et al. Hemithoracic radiotherapy for mesothelioma: Lack of benefit or lack of statistical power? Lancet Oncol 2016;17:e43-e44. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Bueno R, Opitz I. Surgery in Malignant Pleural Mesothelioma. J Thorac Oncol 2018;13:1638-54. [Crossref] [PubMed]
- Shapiro M, Swanson SJ, Wright CD, et al. Predictors of Major Morbidity and Mortality After Pneumonectomy Utilizing The Society for Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg 2010;90:927-34; discussion 934-5. [Crossref] [PubMed]
- Burt BM, Cameron RB, Mollberg NM, et al. Malignant pleural mesothelioma and the Society of Thoracic Surgeons Database: An analysis of surgical morbidity and mortality. J Thorac Cardiovasc Surg 2014;148:30-5. [Crossref] [PubMed]
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13. [Crossref] [PubMed]
- Van Schil PE, Baas P, Gaafar R, et al. Trimodality therapy for malignant pleural mesothelioma: Results from an EORTC phase II multicentre trial. Eur Respir J 2010;36:1362-9. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: Results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6. [Crossref] [PubMed]
- Bovolato P, Casadio C, Billè A, et al. Does surgery improve survival of patients with malignant pleural mesothelioma?: A multicenter retrospective analysis of 1365 consecutive patients. J Thorac Oncol 2014;9:390-6. [Crossref] [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [Crossref] [PubMed]
- Taioli E, Wolf AS, Flores RM. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg 2015;99:472-80. [Crossref] [PubMed]
- Batirel HF, Metintas M, Caglar HB, et al. Adoption of pleurectomy and decortication for malignant mesothelioma leads to similar survival as extrapleural pneumonectomy. J Thorac Cardiovasc Surg 2016;151:478-84. [Crossref] [PubMed]
- Verma V, Ahern CA, Berlind CG, et al. National Cancer Database Report on Pneumonectomy Versus Lung-Sparing Surgery for Malignant Pleural Mesothelioma. J Thorac Oncol 2017;12:1704-14. [Crossref] [PubMed]
- Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: A powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683-90. [Crossref] [PubMed]
- Stokes WA, Bronsert MR, Meguid RA, et al. Post-treatment mortality after surgery and stereotactic body radiotherapy for early-stage non-small-cell lung cancer. J Clin Oncol 2018;36:642-51. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Post-treatment mortality after definitive chemoradiotherapy versus trimodality therapy for locally advanced non-small cell lung cancer. Lung Cancer 2019;127:76-83. [Crossref] [PubMed]
- Verma V, Ahern CA, Berlind CG, et al. Survival by Histologic Subtype of Malignant Pleural Mesothelioma and the Impact of Surgical Resection on Overall Survival. Clin Lung Cancer 2018;19:e901-12. [Crossref] [PubMed]
- Shaaban SG, Verma V, Choi JI, et al. Utilization of Intensity-Modulated Radiation Therapy for Malignant Pleural Mesothelioma in the United States. Clin Lung Cancer 2018;19:e685-92. [Crossref] [PubMed]
- Friedberg JS, Simone CB 2nd, Culligan MJ, et al. Extended Pleurectomy-Decortication-Based Treatment for Advanced Stage Epithelial Mesothelioma Yielding a Median Survival of Nearly Three Years. Ann Thorac Surg 2017;103:912-9. [Crossref] [PubMed]
- Edwards JG, Stewart DJ, Martin-Ucar A, et al. The pattern of lymph node involvement influences outcome after extrapleural pneumonectomy for malignant mesothelioma. J Thorac Cardiovasc Surg 2006;131:981-7. [Crossref] [PubMed]
- Flores RM, Routledge T, Seshan VE, et al. The impact of lymph node station on survival in 348 patients with surgically resected malignant pleural mesothelioma: Implications for revision of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg 2008;136:605-10. [Crossref] [PubMed]
- Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: A Monte Carlo study. Stat Med 2007;26:734-53. [Crossref] [PubMed]
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41-5. [Crossref]
- Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol 2010;172:1092-7. [Crossref] [PubMed]
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. [Crossref] [PubMed]
- Verma V, Wegner RE, Brooks ED, et al. Chemotherapy Versus Supportive Care for Unresected Malignant Pleural Mesothelioma. Clin Lung Cancer 2019;20:263-9. [Crossref] [PubMed]
- Verma V, Ahern CA, Berlind CG, et al. Facility volume and postoperative outcomes for malignant pleural mesothelioma: A National Cancer Data Base analysis. Lung Cancer 2018;120:7-13. [Crossref] [PubMed]
- Verma V, Wegner RE, Ludmir EB, et al. Management of Malignant Pleural Mesothelioma in the Elderly Population. Ann Surg Oncol 2019;26:2357-66. [Crossref] [PubMed]
- Verma V, Sleightholm RL, Rusthoven CG, et al. Malignant Peritoneal Mesothelioma: National Practice Patterns, Outcomes, and Predictors of Survival. Ann Surg Oncol 2018;25:2018-26. [Crossref] [PubMed]
- Barsky AR, Ahern CA, Venigalla S, et al. Gender-based Disparities in Receipt of Care and Survival in Malignant Pleural Mesothelioma. Clin Lung Cancer 2020. [Crossref] [PubMed]