
A multicenter, prospective, observational study on montelukast monotherapy or montelukast-based combinations treating cough variant asthma
Introduction
Cough variant asthma (CVA) is atypical with cough as the predominant symptom and normal pulmonary function associated with airway hyperresponsiveness (1). CVA shares several pathophysiological features with classical asthma (CA) (2-5). The prevalence of CVA is 5–6% in children but unclear in adults (6,7). CVA represents one of the most common causes of chronic cough, accounting for 24–35% of patients in western countries (8,9), 42% in Japan (10) and 32.6% in China (11).
The disease nature of CVA varies. Some patients may progress to CA, whereas others may resolve cough without long-term treatment (6). Inhaled corticosteroids (ICS) is the standard treatment to initiate among CVA patients (4,5,12-14). Cysteinyl leukotriene receptor (CysLTR) antagonists (LTRA) such as montelukast (MONT) or zafirlukast are effective alternative to ICS for CVA (15,16). The Global Initiative for Asthma (GINA) recommended a stepwise algorithm for the long-term asthma control commencing with as-needed low-dose ICS-formoterol or low-dose ICS taken with SABA (controller medication) for symptoms control against mild asthma (17). A step-up by using daily low-dose ICS or LTRA and adding long-acting beta-2-agonists (LABA) or LTRA to ICS, or ICS dose up-titration, is considered if there were uncontrolled symptoms or exacerbations within 2–3 months or any clinical emergency due to exacerbations.
Some evidence showed that CVA causes more psychological and social burdens (12,18) and maybe more challenging to manage than mild CA (19). Small studies examined LTRA as monotherapy treating CVA for 2–4 weeks. Cough and life quality indexes improved significantly with anti-inflammatory effects (15,16,20,21). Treating CVA as severe asthma has also been proposed and investigated (18,22). Nevertheless, this investigation aimed at addressing CVA-associated psychological burdens. China’s 2009 guideline supported LTRA use for CVA but did not specify its treatment algorithm (23). Local clinical practice prescribes less ICS and favours non-steroid medicines for asthma control (24), similarly to other Asian countries (25).
Therefore, we aimed to evaluate the effectiveness of MONT monotherapy or in combination with low-dose ICS +/− LABA for short-term CVA control for Chinese patients in the real-world clinical setting. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1898).
Methods
Study design and setting
This was a multicentre, prospective, observational cohort study (MK-0476-916) in China to evaluate the effectiveness of MONT as monotherapy or in combination with low-dose ICS or low-dose ICS and LABA for CVA in the real-world clinical setting. The enrollment commenced in December 2015 and ended in March 2018 at respiratory clinics from 16 tertiary hospitals. The study followed the routine clinical practice of CVA short-term control. The investigator specialised in respiratory medicine assessed and decided if the patient was appropriate for MONT alone or MONT-based combination therapy and followed up the patient for 4 weeks after the treatment initiation. Merck Sharp & Dohme (MSD) China designed and sponsored the study and analysed the data. The study was approved by the independent ethics committee of all study sites (approval no. 2015-109) and conducted following the guidelines of the International Conference on Harmonization and Good Pharmacoepidemiology Practice (GPP), the Declaration of Helsinki (as revised in 2013) and local regulatory guidance.
Participants
Patients were eligible if they were Chinese aged 18 years or above, had confirmed CVA defined as chronic cough >8 weeks with a positive bronchial challenge test and normal chest X-ray findings and normal spirometry, and indicated for MONT treatment as the investigator’s discretions. Patients were excluded if they had impaired pulmonary function as measured by spirometry [forced expiratory volume in 1 second (FEV1)/(forced vital capacity (FVC) <70%], had been smoking >10 pack-years, had other diseases causing chronic cough [e.g., bronchitis, lung cancer, left ventricular dysfunction, psychologic or angiotensin-converting enzyme (ACE) inhibitor-induced cough], had received any therapy for CVA within 28 days before the study inclusion (e.g., ICS, LABA, theophylline, or a LTRA other than MONT), or were pregnant or breastfeeding. All patients provided written informed consent before study screening.
Study procedure and assessments
The investigator screened patients based on findings from medical history and laboratory and radiological assessments at the initial study visit (baseline). The investigator decided the treatment option against the patient’s cough symptoms and comorbidities, including allergic rhinitis. As in normal clinical practice, the investigator and the patient were both aware of the treatment scheme allocated. The patient used a paper-formed diary to record the severity of cough, SABA use (in every 12 hours), and change or discontinuation of study medications for 4 weeks. The investigator reviewed diary information and assessed the patient every 1 or 2 weeks for treatment effectiveness. Also, the patient was instructed to complete the self-administered Leicester Cough Questionnaire (LCQ) at the site every 2 weeks. Fractional exhaled nitric oxide (FeNO) and sputum eosinophil counts were done at the initial visit and the end of the study for patients who were willing to accept. The patient may discontinue by consent withdrawal or based on the investigator’s decision and was not allowed to re-enroll.
Cough score (CS) and LCQ
CS is a verified tool recommended by Chinese Guideline (23) for diagnosis and treatment of cough to measure cough-specific symptoms. This scoring system reflects cough frequency and influence on life quality by a simple and quantifiable index (grades 0–3) for daytime, nighttime and daily total (Table S1). The local clinical practice considers 25% of CS reduction as clinically significant.
LCQ is a 19-item self-administered quality of life measure of chronic cough which is responsive to change (26). Before the study execution, LCQ was translated into Chinese language and validated for its accuracy for CVA patients.
Exposure and outcome measures
MONT was initiated at 10 mg daily as monotherapy or in combination with low-dose ICS (included beclomethasone 100–200 mg, budesonide 200–400 mg or another ICS as appropriate) or low-dose ICS and LABA (i.e., fixed-dose formulations of budesonide/formoterol). The investigator may adjust the regimen to achieve asthma control. The extent of exposure by counting daily doses was not documented.
Treatment outcome was primarily measured as changes in CS at the end of each week from baseline and the proportion of patients who had a reduction in CS >25% at the end of each week from baseline. Weekly asthma control, as a real-world effectiveness outcome, was defined, if the patient met all following criteria at each week: (I) no more than 2 days of daytime cough (CS >1); (II) no any night sleep disturbance by cough (CS >1); (III) no changes in observed treatment regimen; and (IV) no significant SABA use (≥2 times) (17). Secondary analyses were performed to assess cough-free days and nights (CS =0), the patient’s LCQ, and SABA user (if weekly SABA ≥2 times).
Statistical analysis
Sample size consideration
The sample size was calculated by achieving the precision of estimation [two-sided 95% confidence interval (CI)] by 5% of the mean change in CS at the end of the study from baseline by MONT alone or two MONT-based combinations. Literature has suggested such mean (SD) changes are 1.5 (0.4), 2.5 (0.4), respectively (27,28). Therefore, a sample size of 112 achieved a two-sided precision of 0.075 of the point estimate for the monotherapy group; a sample size of 42 achieved a two-sided precision of 0.125 of the point estimate for each of the two combination groups. Assuming 20% of patients who had premature discontinuation, a total sample size of 240 patients (MONT monotherapy: 140, MONT + ICS: 50, and MONT + ICS/LABA: 50, respectively) were planned for the study.
Analysis population
The efficacy analysis population included patients who had at least one dose of MONT and any post-baseline study observations. Two analysis subsets were defined to include and analyze patients who had CS since the baseline visit. The CS evaluable population included patients who received at least one dose of MONT and documented CSs >2 days in the first week of the study. The CS per protocol population included patients who received at least one dose of MONT, had no change in treatment regimen and recorded CSs >2 days in each week during the study. Outcome measures including CS and weekly asthma control were analyzed in the CS evaluable and the CS per protocol populations.
Analysis of endpoints
Changes in CS from baseline were calculated as CS at the end of each week minus that at the baseline. Mean (SD) and corresponding 95% CIs were given under t-distribution of the sample. The mean change in CS from baseline was considered statistically significant if its 95% CI does not contain 0. Total CS reduction (>25%) and weekly asthma control were presented as the number and percentage. Corresponding 95% CI for the proportion was estimated based on exact (Clopper-Pearson) method. For the primary analysis of CS changes, a between-group statistical comparison was not made, which avoided producing potential incorrect statistical conclusion favouring one particular treatment group. A post-hoc Chi-square test was made to compare asthma control between MONT alone versus MONT-based combination (pooled by two combination groups). Baseline CS was defined as the CS collected on the date of the initial visit. CS at the end of each week was defined as the last non-missing value in the week. If CS collected were ≤2 days in each week, the highest CS in the previous 7 days were carried forward to impute the missing data throughout the week. For patients who had change in treatment regimen, the highest CS in the previous 7 days were carried forward to all subsequent CS data points throughout the study. These patients were analysed in the original treatment exposure.
Secondary analyses are descriptive. The study created a multivariate logistic regression model to explore the association between asthma control at the end of the study and clinical characteristics. The model displays all selected variables with an adjusted odds ratio (OR) with corresponding 95% CI and P value by a Wald test. Statistical analyses were performed using SAS 9.4 and SAS JMP 13.0 (SAS Institute, Cary, NC, USA). P value of 0.05 was considered as statistically significant whenever applicable.
Results
Patients disposition and demographics characteristics
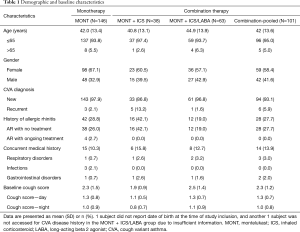
There were 247 patients enrolled (MONT =146, MONT + ICS =38, MONT+ ICS/LABA =63) and 197 patients (79.8%) included in the study, among which 211 (85.4%) were in the CS evaluable population and 177 (71.7%) were in the CS per protocol population (Figure 1). The enrollment ended in March 2018 as there was no potential to enroll more patients in the MONT + ICS group. Among 146 patients in the MONT group, 120 completed the study procedures, and 26 discontinued prematurely (13 withdrew consent, 11 had lost-to-follow-up, and 2 were removed from the study by the investigator, respectively). The reasons for premature discontinuation in the MONT + ICS and MONT + ICS/LABA groups were similar to those in the MONT group. A total of 131 patients were assessed having efficacy-related data in the MONT group, but 103 patients were finally included in the CS per protocol population for analysis. Among 28 patients who did not meet the definition to enter the CS per protocol population, 14 had their treatment switched to combination therapies, 6 did not give complete CS diary; 4 had consent withdrawal during the treatment, 3 had LCQ data only, and 1 were removed by the investigator from the study. There were 33 and 54 patients who were assessed having efficacy-related data in the MONT + ICS and MONT + ICS/LABA groups, respectively; 31 and 43 were finally included in the CS per protocol population in these two groups.

Table 1 shows the patient’s baseline characteristics. Patients were middle-aged adults (mean age 42.0–44.9 years) and most CVA patients were newly diagnosed (>85%). The proportion of patients with allergic rhinitis was higher in the MONT + ICS group [42.1% (16/38)]. Baseline CS presented as total, daytime, and nighttime did not differ significantly among the three treatment groups.
Full table
CS and asthma control
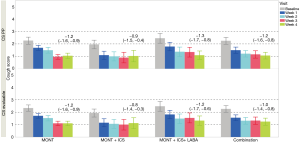
The mean change (95% CI) in total CS at the end of the study from baseline was −1.2 (−1.6, −0.9) in the MONT group, −0.9 (−1.5, −0.4), and −1.3 (−1.7, −0.8) in the MONT + ICS and MONT + ICS/LABA groups in the CS per protocol population. CS reductions were statistically significant among all groups (95% CIs do not include 0, Figure 2). The analysis in the CS evaluable population showed similar results. The frequency distribution of patients’ total CS over time supported the trend in CS reduction over time (Figure S1).
In the CS per protocol population, the proportion of patients who had a CS reduction >25% from baseline at the end of the study [78.6% (81/103), 95% CI: 69.5%, 86.1%] was numerically higher than that at week 2 [67.0% (69/103), 95% CI: 57.0%, 75.9%] in the MONT group (Figure 3); proportions of patients who had a CS reduction >25% from baseline were numerically comparable between the two combination groups at weeks 2 and 4 (Figure 3).
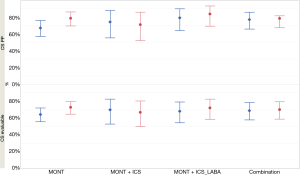
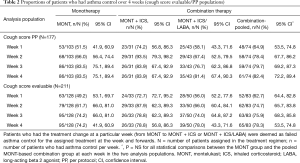
Table 2 presents weekly asthma control. In the CS per protocol population, the MONT group showed a moderate CVA control at weeks 1 and 2 [51.5% (53/103), 95% CI: 41.9%, 60.9%; 66.0% (68/103), 95% CI: 56.4%, 74.4%] and a markedly high rate of asthma control at the end of study [83.5% (86/103), 95% CI: 75.1%, 89.4%]. In the CS evaluable population, analyses suggested a similar trend in the MONT group. The MONT + ICS group had rates of asthma control between 72.7% (24/33, 95% CI: 55.8%, 84.9%) and 83.9% (26/31, 95% CI: 67.4%, 92.9%) during the study in the two analysis populations, where rates in the MONT + ICS/LABA group were numerically lower. There was no significant difference in the rate of weekly asthma control between the MONT group and MONT-based combination in the two analysis populations.
Full table
Secondary analyses
As shown in Table 3, weekly cough-free days (3.4–3.6) and nights (4.5–4.8) remained consistent from week 2 in the MONT + ICS group and was the highest; whereas the MONT and MONT + ICS/LABA groups had numerically comparable cough-free days and nights. At week 4, cough-free days and nights were more than those at week 1 within the three treatment groups, respectively. As the 95% CIs for cough-free days and nights were overlapped between any two treatment groups at week 4, there was no statistical difference among three treatment groups for any between-group statistical comparison.
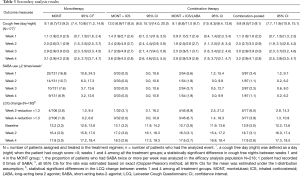
Full table
At week 1, the proportion of patients who had SABA ≥2 times a week was 16.8% (22/131, 95% CI: 10.8%, 24.3%), 5.6% (3/54, 95% CI: 1.2%, 15.4%) in the MONT and the MONT + ICS/LABA groups, respectively. SABA use decreased overtime during the 4-week observation. At week 4, the proportion of patients who had SABA ≥2 a week was 6.9% (9/131, 95% CI: 3.2%, 12.6%), 1.9% (1/54, 95% CI: 0.0%, 9.9%) in the MONT and the MONT + ICS/LABA groups, respectively. No patient used SABA ≥2 times in the MONT + ICS group during the study.
Also, there was an increase in LCQ scores over time in three treatment groups during the study. At week 4, the proportion of patients who had a reduction >1.3 in LCQ was 1.9% (2/106, 95% CI: 0.2%, 6.6%), 0.0% (0/32, 95% CI: 0.0%, 10.9%), and 6.7% (3/45, 95% CI: 1.4%, 18.3%), in the MONT, the MONT + ICS, and the MONT + ICS/LABA groups, respectively.
Predictors for asthma control
Multivariate analysis on predictors for asthma control at the end of the study was performed in the CS evaluable population (Table 4). Adjusted for variables, treatment option (MONT vs. MONT-based combinations) was not a significant predictor for CVA control at the end of the study (OR: 0.97, 95% CI: 0.47, 1.98, P=0.94). Allergic rhinitis was inversely associated with asthma control at the end of the study (OR: 0.52, 95% CI: 0.25, 1.08, P=0.07). Early asthma control (at week 1) resulted in a 4.60-fold likelihood in achieving asthma control at the end of the study (OR: 4.60, 95% CI: 2.30, 9.64, P<0.0001).
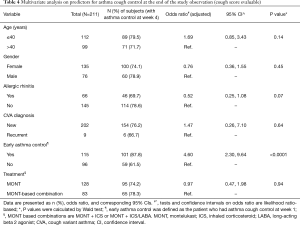
Full table
Discussion
Despite global evidence on asthma control (29,30), clinical investigations on CVA are few and limited to small sample size (15,16,20,21,31-36). This study, however, had a relatively larger sample size to evaluate the short-term control of CVA by MONT-based regimens as initial treatment in the real-world practice. The study warranted CVA diagnosis by clinical, spirometric and radiological findings based on guideline-recommended criteria and differentiate comorbidities from CVA diagnosis. Study findings suggested that MONT alone or in combination with low-dose ICS or low-dose ICS plus LABA significantly reduced CS and had an improvement in asthma control and considerable cough-free days at the end of 4-week study observation.
Uncontrolled CVA interferes with sleep, physical, and social activities across all age groups. CVA was shown to cause higher depression levels compared with CA (18) and progress to CA (6,19). Cough control to destress patients and delink classic asthma is thus necessary. Studies have indicated that 1–2 weeks of therapy by bronchodilator, ICS (6) or MONT (15,16,20,21) improved cough symptoms. In this study, CS reduced remarkably since week 2 and most patients had CS reduction>25% at the end of the study in all treatment groups. Most short-term studies measured and compared cough on scale or scores (15,16,20,21). However, our study defined weekly asthma control using an approach to reflect a real-world clinical setting. MONT monotherapy exhibited an improvement in asthma control after three weeks of treatment. When adding confounding (treatment switch, SABA use, or missing CS) to weekly asthma control, rates of asthma control did not decrease dramatically. One interesting finding is that the triple therapy had a similar rate of weekly asthma control compared with MONT monotherapy. This finding differed from one Canadian study where add-on MONT to ICS/LABA was more effective among asthma patients with allergic rhinitis (37). In addition, MONT-based combinations did not show significantly better asthma control. One Japanese study proposed CVA treatment as severe CA to achieve symptom control (18) and thus, may favour combination therapies. In this study, however, the proportion of patients who had allergic rhinitis was higher in the MONT + ICS group, which may affect the effectiveness of the combination therapy. Also, current evidence indicates that the additive effect of LTRA is very limited to the effects of ICS combination treatment on CVA patients. Our findings merit further investigations.
As CS significantly decreased, approximately 3 or 4 cough-free days and nights have been noted after 2 weeks of treatment in all treatment groups. One study in the 1990s found that CVA patients were free of cough after a median follow-up of 28 months (12). Our results showed a promising trend to eliminate cough in the short term. LCQ analysis suggested an overall improvement in scores from three domains, which confirmed that addressing cough episodes destresses patients. MONT monotherapy had a higher proportion of SABA ≥2 times each week during the study. Patients may choose to self-administer more ICS or ICS/LABA as relief medications for cough and related stress as needed. The study did not collect complete treatment compliance. It is not clear if occasional dose titrations of ICS or ICS/LABA replaced SABA use for better symptom control. Finally, multivariate logistic regression suggested achieving early asthma control (week 1) was a strong predictor for asthma control at the end of the study. Clinical relevance of allergic rhinitis to CVA has been established by increasing eosinophilic lower airway inflammation (38). Our results indicated that patients with allergic rhinitis were less likely to achieve asthma control at the end of the study, but this association is not statistically significant. A MONT-based combination did not result in a better chance of having asthma control at the end of the study.
This study has limitations. Firstly, this study did not randomise and compare treatments. Adding a placebo group to compare treatments is challenging in real-world clinical practice. The magnitude of treatment effectiveness as measured by CS cannot exclude the placebo effect. In addition, the investigator had a selection bias on the treatment regimen, and the patient had an assessment bias on the study’s questionnaire. These biases may influence the study results. Secondly, the study was initially designed to assess the quantitative measures, including the CS and LCQ within four weeks. Long-term CVA control was not measured. Also, due to a difficulty in assessing drug accountability in a real-world setting, the extent of treatment compliance associated with the outcomes was not studied. Last, the generalizability of study results needs caution because treatment effectiveness may vary due to different symptom severity and outcome measures in real-world practice.
Conclusions
In conclusion, the effectiveness of MONT alone or in combination with ICS or ICS and LABA was acceptable for CVA short-term control in clinical practice.
Acknowledgments
The study team acknowledged the contributions of all the investigators in collecting study data.
Funding: This work was supported by Merck Sharp & Dohme China, Shanghai, China.
Footnote
Provenance and Peer Review: This article was a free submission to the journal. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1989
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1989
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1989). LC, JW and AM are employees of MSD China, Shanghai, China. All other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the independent ethics committee of all study sites (approval no. 2015-109) and conducted following the guidelines of the International Conference on Harmonization and Good Pharmacoepidemiology Practice (GPP), the Declaration of Helsinki (as revised in 2013) and local regulatory guidance. All patients provided written informed consent before study screening.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Corrao WM, Braman SS, Irwin RS. Chronic cough as the sole presenting manifestation of bronchial asthma. N Engl J Med 1979;300:633-7. [Crossref] [PubMed]
- Takemura M, Niimi A, Matsumoto H, et al. Atopic features of cough variant asthma and classic asthma with wheezing. Clin Exp Allergy 2007;37:1833-9. [Crossref] [PubMed]
- Matsumoto H, Niimi A, Takemura M, et al. Features of cough variant asthma and classic asthma during methacholine-induced brochoconstriction: a cross-sectional study. Cough 2009;5:3. [Crossref] [PubMed]
- Niimi A, Amitani R, Suzuki K, et al. Eosinophilic inflammation in cough variant asthma. Eur Respir J 1998;11:1064-9. [Crossref] [PubMed]
- Niimi A, Matsumoto H, Minakuchi M, et al. Airway remodelling in cough-variant asthma. Lancet 2000;356:564-5. [Crossref] [PubMed]
- Johnson D, Osborn LM. Cough variant asthma: a review of the clinical literature. J Asthma 1991;28:85-90. [Crossref] [PubMed]
- Iyer VN, Lim KG. Chronic cough: an update. Mayo Clin Proc 2013;88:1115-26. [Crossref] [PubMed]
- Poe RH, Harder RV, Israel RH, et al. Chronic persistent cough. Experience in diagnosis and outcome using an anatomic diagnostic protocol. Chest 1989;95:723-8. [Crossref] [PubMed]
- McGarvey LP, Heaney LG, Lawson JT, et al. Evaluation and outcome of patients with chronic non-productive cough using a comprehensive diagnostic protocol. Thorax 1998;53:738-43. [Crossref] [PubMed]
- Shirahata K, Fujimoto K, Arioka H, et al. Prevalence and clinical features of cough variant asthma in a general internal medicine outpatient clinic in Japan. Respirology 2005;10:354-8. [Crossref] [PubMed]
- Lai K, Chen R, Lin J, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest 2013;143:613-20. [Crossref] [PubMed]
- Cheriyan S, Greenberger PA, Patterson R. Outcome of cough variant asthma treated with inhaled steroids. Ann Allergy 1994;73:478-80. [PubMed]
- Niimi A, Torrego A, Nicholson AG, et al. Nature of airway inflammation and remodeling in chronic cough. J Allergy Clin Immunol 2005;116:565-70. [Crossref] [PubMed]
- Irwin RS, Ownbey R, Cagle PT, et al. Interpreting the histopathology of chronic cough: a prospective, controlled, comparative study. Chest 2006;130:362-70. [Crossref] [PubMed]
- Dicpinigaitis PV, Dobkin JB, Reichel J. Antitussive effect of the leukotriene receptor antagonist zafirlukast in subjects with cough-variant asthma. J Asthma 2002;39:291-7. [Crossref] [PubMed]
- Spector SL, Tan RA. Effectiveness of montelukast in the treatment of cough variant asthma. Ann Allergy Asthma Immunol 2004;93:232-6. [Crossref] [PubMed]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2018. Available online: accessed March 26, 2019.https://ginasthma.org/
- Saito N, Itoga M, Tamaki M, et al. Cough variant asthma patients are more depressed and anxious than classic asthma patients. J Psychosom Res 2015;79:18-26. [Crossref] [PubMed]
- Niimi A. Cough and Asthma. Curr Respir Med Rev 2011;7:47-54. [Crossref] [PubMed]
- Kawai S, Baba K, Matsubara A, et al. The efficacy of montelukast and airway mast cell profiles in patients with cough variant asthma. J Asthma 2008;45:243-50. [Crossref] [PubMed]
- Takemura M, Niimi A, Matsumoto H, et al. Clinical, physiological and anti-inflammatory effect of montelukast in patients with cough variant asthma. Respiration 2012;83:308-15. [Crossref] [PubMed]
- Effects of Leukotriene Modulator Montelukast on Cough Variant Asthma. Available online: accessed May 2019.https://clinicaltrials.gov/ct2/show/study/NCT01404013
- Lai K. Chinese National Guidelines on Diagnosis and Management of Cough: consensus and controversy. J Thorac Dis 2014;6:S683-8. [PubMed]
- Ip KI, Hon KL, Tsang KYC, et al. Steroid phobia, Chinese medicine and asthma control. Clin Respir J 2018;12:1559-64. [Crossref] [PubMed]
- Morishima T, Otsubo T, Gotou E, et al. Physician adherence to asthma treatment guidelines in Japan: focus on inhaled corticosteroids. J Eval Clin Pract 2013;19:223-9. [Crossref] [PubMed]
- Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003;58:339-43. [Crossref] [PubMed]
- Xie J, Wang Y, Zhang Z. Radom Compare Study of Efficacy of Tulobuterol Tape and Montelukast-na on Children's Cough Variant Asthma. Medical Information 2013;26:247-8.
- Zheng L, Jiang X, Ding L, et al. The clinical observation of cough variant asthma on Suhuang Zhike Capsule combined Seretide treatment. Journal of Emergency in Traditional Chinese Medicine 2014;1:23.
- O'Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled Combined Budesonide-Formoterol as Needed in Mild Asthma. N Engl J Med 2018;378:1865-76. [Crossref] [PubMed]
- Bateman ED, Reddel HK, O'Byrne PM, et al. As-Needed Budesonide-Formoterol versus Maintenance Budesonide in Mild Asthma. N Engl J Med 2018;378:1877-87. [Crossref] [PubMed]
- Wang XP, Yang LD, Zhou JF. Montelukast and budesonide combination for children with chronic cough-variant asthma. Medicine (Baltimore) 2018;97:e11557. [Crossref] [PubMed]
- Tagaya E, Kondo M, Kirishi S, et al. Effects of regular treatment with combination of salmeterol/fluticasone propionate and salmeterol alone in cough variant asthma. J Asthma 2015;52:512-8. [Crossref] [PubMed]
- Zhou X, Hong J, Cheng H, et al. Budesonide suspension nebulization treatment in Chinese pediatric patients with cough variant asthma: a multi-center observational study. J Asthma 2016;53:532-7. [Crossref] [PubMed]
- Bao W, Chen Q, Lin Y, et al. Efficacy of procaterol combined with inhaled budesonide for treatment of cough-variant asthma. Respirology 2013;18 Suppl 3:53-61. [Crossref] [PubMed]
- Miao Q, Wei PC, Fan MR, et al. Clinical study on treatment of cough variant asthma by Chinese medicine. Chin J Integr Med 2013;19:539-45. [Crossref] [PubMed]
- Cao Y, Lin SH, Zhu D, et al. WeChat Public Account Use Improves Clinical Control of Cough-Variant Asthma: A Randomized Controlled Trial. Med Sci Monit 2018;24:1524-32. [Crossref] [PubMed]
- Keith PK, Koch C, Djandji M, et al. Montelukast as add-on therapy with inhaled corticosteroids alone or inhaled corticosteroids and long-acting beta-2-agonists in the management of patients diagnosed with asthma and concurrent allergic rhinitis (the RADAR trial). Can Respir J 2009;16 Suppl A:17A-31A.
- Tajiri T, Niimi A, Matsumoto H, et al. Prevalence and clinical relevance of allergic rhinitis in patients with classic asthma and cough variant asthma. Respiration 2014;87:211-8. [Crossref] [PubMed]


