Obstructive sleep apnea is a common disorder in the population—a review on the epidemiology of sleep apnea
Introduction
Obstructive sleep apnea (OSA) is characterized by recurrent episodes of partial and complete airway obstructions during sleep with repetitive apneas and hypopneas as a result (1). The disease severity is measured using the apnea-hypopnea index (AHI), i.e., the mean number of apneas and hypopneas per hour of sleep. OSA is defined when the AHI is ≥5 and OSA syndrome when AHI ≥5 is accompanied with daytime sleepiness (1). The American Association of Sleep Medicine defined daytime sleepiness as mild, moderate and severe in relation to impact on social life during the daytime (1). The Epworth Sleepiness Scale (ESS) is, however, the most often used measure to define daytime sleepiness (2,3). Diagnostic equipment and definitions of oxygen desaturations, apnea, hypopnea, OSA and daytime sleepiness has, changed over time, which in turn affects estimates of the prevalence of sleep apnea.
In the first prevalence surveys when sleep apnea was considered a rare disorder, sleep recordings were only performed in sub-samples with a high risk of OSA in a first-stage screening procedure, and the estimated prevalence in the whole population was based on the assumption that there was no OSA at all among the remaining participants. The estimated prevalence of OSA syndrome in these studies ranged from 0.7% to 3.3% (4-8).
Patients in sleep clinic cohorts have all been referred for the diagnostic sleep test because of symptoms suggestive of the diagnosis and they are most frequently heavy snorers suffering from daytime sleepiness. Epidemiological studies on sleep apnea will identify all subjects with OSA defined as AHI ≥5. However, only part of them will have symptoms such as snoring and daytime sleepiness reflecting subjects eligible for sleep apnea investigation on clinical grounds. This article reviews the epidemiology of OSA on prevalence and associated factors including possible risk factors and consequences.
Prevalence of OSA
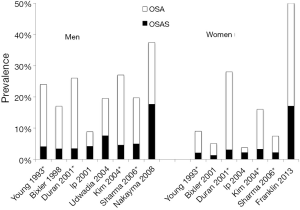
We identified eleven population-based epidemiological studies from US, Chine, Spain, India, Korea, Japan and Sweden published between 1993 and 2013. All eleven studies were done in two stages. In stage one, they send postal questionnaires to a random sample of the population. In stage two, they investigated a random sample of responders from stage one, with oversampling of subjects who reported snoring and daytime sleepiness and then weighted their results to the population. The prevalence of OSA defined at an AHI ≥5 were a mean of 22% (range, 9-37%) in men and 17% (range, 4-50%) in women (Table 1, Figure 1) (3,8-17). OSA syndrome defined as apnea-hypopnea index ≥5 and excessive daytime sleepiness occurred in 6% (range, 3-18%) of men and in 4% (range, 1-17%) of women (Figure 1). The prevalence in different studies has increased with time and OSA in the last studies was reported in 37% of men and in 50% of women (3,16). The differences over time could be due to different equipment and definitions for the apnea-hypopnea scoring. There are also differences in study design and populations. The results may also be affected by an increased amount of obese subject due to the obesity epidemic.
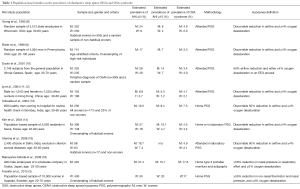
Full table
Associated factors with OSA
Gender
OSA is more common in men than women. The male-to-female ratio is estimated to about 2:1 in the general population (Table 1, Figure 1) and the prevalence of snoring shows similar gender differences (18-20). The male predominance is higher in clinical populations (21,22). Possible explanations for the male predominance include hormonal effects on upper airway muscles and collapsibility, gender differences in body fat distribution and differences in pharyngeal anatomy and function. Hormonal influences could play an important role in the pathogenesis of OSA, as the prevalence seems to be higher in post- versus pre-menopausal women (17,23). The pathophysiological roles of hormones are, however, unclear and the gender differences in prevalence remained also in the elderly (24).
A recent study, by Franklin et al., reported that sleep apnea occurs in as much as 50% of females aged 20-70 years old in the population (3). There was no relationship between OSA and daytime sleepiness in this study. Instead hypertension, obesity and age were associated with sleep apnea in females. It is, thus, possible that sleep apnea has not been observed as a public heath problem in females, as they have other signs of sleep apnea than males.
Age
Snoring frequency increases with age up to 50 to 60 years old and then decrease in both men and women (6,18,25,26). The prevalence of OSA also increases with age independent of other risk factors including obesity (3,8,27,28). On the contrary to snoring, the prevalence of OSA still increases also after the age of 60 years (3,8,10). Bixler et al. reported an increase in OSA after 65 years but the frequency of OSA syndrome declined (8). The above findings indicate that self-reported snoring and doctor-diagnosed OSA syndrome display similar age distributions with a decline at older ages in contrast to the age distribution of OSA with an AHI over five that increase with age also in the elderly.
Several studies have reported little or no association between sleep-disordered breathing and morbidity and mortality at older ages, and it has been suggested that sleep apnea in seniors represents a specific entity compared with middle-aged adults (29).
Obesity
Obesity is a major risk factor for snoring and sleep apnea and a majority of patients with OSA are overweight (3,30-32). Caloric restriction or bariatric surgery reduces the severity of sleep apnea (28,33-36). One randomized controlled study reported a decrease in AHI using very low calorie diet (37). Another recent study reported that despite an effect of diet on AHI compared with continuous positive airway pressure (CPAP), patients were still better off with the combination of diet and CPAP than with CPAP alone (38). Men are more likely than women to increase their AHI at a given weight gain regardless of starting weight, waist circumference, age, or ethnicity (39).
Obesity is believed to predispose to OSA because of mass loading in the upper airway (40). Controversy remains whether specific measures of body habitus, such as neck or waist circumference, are better predictors of sleep-disordered breathing as compared with body mass index (BMI) alone. Neck circumference was in a population-based sample more important as a risk factor for snoring with increasing BMI in obese than in lean women (26).
Young et al. estimated that 58% of moderate to severe cases of OSA is due to a BMI of ≥25 kg/m2 (34). This highlights the need for effective strategies to implement long-term weight-loss programs to prevent OSA and the ongoing epidemics of obesity. Not only subjects with obesity and fat necks suffer from sleep apnea, but also lean subject and about one-third of OSA syndrome patients are non-obese (41). Franklin et al. reported that 39% of normal weighted women had OSA but only 0.1% of them had severe sleep apnea (3).
Smoking
Several cross-sectional epidemiological surveys observed significant associations between cigarette smoking and snoring or sleep apnea (18,19,42-46). Possible underlying mechanisms include airway inflammation and sleep instability from overnight nicotine withdrawal (47). Never-smokers who have been exposed to passive smoking on a daily basis display an increase in the odds of being a habitual snorer of 1.6 (95% CI, 1.2-2.1) after adjusting for age and BMI according to the Respiratory Health in Northern Europe Study (44). In a Swedish longitudinal study, smoking predicted the development of snoring in men younger than 60 years old but not in older ones (25).
Wetter et al. found a dose-response relationship between smoking and the severity of sleep apnea. Heavy smokers ran the greatest risk, while former smoking was unrelated to snoring and sleep-disordered breathing after adjustment for confounders (46). Smoking is, however, not an established risk factor for OSA. In the analysis from the Sleep Heart Health Study, smokers actually displayed less sleep apnea than non-smokers and there are still no available data on the impact of smoking on the incidence and remission of sleep apnea (48).
Alcohol
Alcohol intake reduces motor output to the upper airways with hypotonia of the oropharyngeal muscles as a result (49). In studies performed in the laboratory, alcohol increases both the number of apneas and the duration of apnea (50,51). The results did, however, diverge, when the relationship between chronic alcohol use and snoring or sleep apnea was analyzed in epidemiological studies and an association was found by some but not by others (13,25,30,52-54). Svensson et al. reported that alcohol dependence was only related to snoring in lean women with a BMI of <20 kg/m2 (26). It is thus possible that the alcohol-induced reduction in motor output to the upper airways is more important in lean women without compromised upper airways from fat deposits and overweight.
Excessive daytime sleepiness
Excessive daytime sleepiness is regarded as the most common and most important symptom of OSA. Numerous randomized controlled trials have demonstrated a significant improvement in daytime sleepiness when such patients are effectively treated with CPAP as compared to sham CPAP or oral placebo (55-60).
Daytime sleepiness is related to OSA and snoring in the general population studies (9,61-63). In the Wisconsin Sleep Cohort Study, about 23% of the women with an AHI of ≥5 reported excessive daytime sleepiness compared with only 10% of non-snoring women (9). The corresponding prevalence in men was 16% and 3% respectively. Similar findings were reported from the Sleep Heart Health Study using the ESS with a significant, progressive increase in sleepiness with increasing AHI in both older and younger subjects and independent of gender, age and BMI (61).
The evidence of apnea induced daytime sleepiness is, however, weak as only a fraction of patients with OSA in the population report daytime sleepiness. Attempts to find the suggested association between the arousals and sleepiness have also failed (64,65). Daytime sleepiness can be due to a number of factors and OSA patients may have suffered from other disorders of sleepiness than sleep apneas. Svensson et al. reported that snoring, but not sleep apnea (AHI >15) was related to excessive daytime sleepiness (63). The association between OSA and sleepiness is also less evident in patients with chronic disease such as in patients with congestive heart failure who report less daytime hypersomnolence regardless of whether they have OSA or not (66). Sleepiness is also frequently reported in the absence of OSA in elderly people and in patients with end-stage renal disease (67,68).
Hypertension
Sleep apnea and hypertension are both prevalent in the community and many individuals suffer from both. Several large population-based, cross-sectional studies reported an independent association between the two conditions (3,10,69-72). Self-reported snoring is also a predictor of developing hypertension in both males and females (43,73,74). Peppard et al. analyzed the odds ratios for the presence of hypertension at a 4-year follow-up among 709 middle-aged participants in the Wisconsin cohort, all of who had been investigated with polysomnography at baseline. Compared with subjects with no OSA, the adjusted odds ratio for prevalent hypertension at follow-up was 2.03 (95% CI, 1.29-3.17) for mild OSA (AHI, 5-14.9) and 2.89 (95% CI, 1.46-5.64) for moderate to severe OSA (AHI ≥15) (75). The same group also provided data from a sub-group who were followed-up after a mean of 7 years using 24-hour blood pressure studies. Regardless of confounders including baseline blood pressure and progress of sleep apnea, there was a significant dose-response relationship between the severity of sleep apnea at baseline and the risk of developing systolic non-dipping blood pressure during sleep (76).
The impact of snoring and OSA on hypertension is less pronounced in overweight and obese subjects when compared with normal-weights in population-based samples (43,69,71). Analyzed by age group, there is an independent relationship of snoring or OSA on hypertension among young and middle-aged participants, but not in the elderly (71-73,77,78). An AHI of ≥15 was independently associated with hypertension in subjects aged <60 years, with an adjusted odds ratio of 2.38 (95% CI, 1.30-4.38), among 6,120 participants in the Sleep Heart Health Study, while no such relationship was found between sleep apnea and hypertension among subjects above that age (72).
Although observational studies indicate a causal relationship between OSA and hypertension the effectiveness of reducing blood pressure by treating OSA is less clear and intervention studies using CPAP have produced mixed results (79).
Coronary artery disease
OSA frequently coexists, but is usually being undiagnosed in patients with cardiovascular disease and several cross-sectional studies support a strong association between OSA and prevalent coronary artery disease, defined as myocardial infarction and/or angina pectoris (80-82). However, sleep apnea was assessed after coronary artery disease was established in the cited studies and thereby limits the conclusion on an etiologic relationship. Cross-sectional epidemiologic studies on self-reported coronary artery disease and snoring or objectively measured OSA have reported a positive association, although of considerably smaller magnitude than that observed in case-control studies (18,83). Among 6,424 participants who underwent in-home polysomnography in the Sleep Heart Health Study, Shahar et al. reported that subjects with the highest quartile of AHI >11 had an adjusted odds ratio of 1.27 of self-reported coronary artery disease after adjusting for confounders including hypertension (84). The relative high age with a mean of 64 years old of participants at study start could be an explanation to the rather modest association.
Patients with OSA had a higher incidence of coronary artery disease (16.2%) compared with snorers without OSA (5.4%) in a prospective study over 7 years (85). Efficient treatment with CPAP significantly reduced the risk of adverse cardiovascular outcomes both when it comes to primary and secondary prevention (85-87).
Population-based prospective studies on sleep apnea and incidence of coronary artery disease are still lacking.
Stroke
Clinical cohorts suggest an important link between sleep apnea and stroke. Spriggs et al. followed patients with recent stroke until death or 6 months and found that previous stroke and regular snoring were the only two risk factors that adversely affected mortality (88). Yaggi et al. followed 1,022 patients being investigated on clinical grounds an concluded that OSA syndrome significantly increases the risk of stroke or death from any cause, and the increase is independent of other risk factors, including hypertension (89). Valham et al. found a dose-response relationship between AHI at baseline among patients with coronary artery disease and the incidence of stroke during a 10-year follow-up after adjusting for potential confounders (90). Moreover, in stroke survivors, the occurrence of OSA, but not central sleep apnea, was a significant predictor of early death (91).
Population based studies also support the evidence of stroke due to OSA. Munoz et al. reported that severe sleep apnea (AHI ≥30) at baseline was associated with a significantly increased risk of developing an ischemic stroke (adjusted hazard ratio 2.52, 95% CI, 1.04-6.01) from a 6-year longitudinal study of a population-based cohort, initially event-free subjects aged 70-100 years (92). Arzt et al. investigated a younger population-based cohort of 1,189 subjects, mean age 47 years with polysomnography. During the following 4 years, 14 subjects suffered a first-ever stroke and this was related to sleep apnea defined as an AHI of ≥20 at baseline, although the association did not reach statistical significance after adjusting for age, gender and BMI (adjusted OR 3.08, 95% CI, 0.74-12.81) (93).
Diabetes mellitus
Sleep-disordered breathing and diabetes mellitus share several risk factors. Insulin resistance and/or type 2 diabetes mellitus coexist with snoring or sleep apnea in general population cross-sectional studies independent of obesity and other confounders (94-101). Furthermore, an independent association between self-reported snoring and incident diabetes is reported in both males and females (102,103).
Longitudinal studies on OSA as a risk factor for future diabetes mellitus have not been conclusive. Among 1,387 participants in the Wisconsin Sleep Cohort, subjects with an AHI ≥15 did not differ significantly from those with an AHI of <5 when it came to the risk of developing diabetes mellitus over a 4-year period (OR 1.62; 95% CI, 0.7-3.6) when adjusting for age, gender, and body habitus (99). Similar findings were reported from the Busselton health study (104). On the contrary, Botros et al. found an independent association between sleep apnea at baseline and incident diabetes in an observational cohort study including 1,233 consecutive patients without diabetes (105). Also in a long-term follow-up of a community-based sample of men there was an independent association between oxygen desaturation index >5 at baseline and incident diabetes mellitus at follow-up after 11 years (OR 4.4; 95% CI, 1.1-18.1), after adjusting for age, BMI, and hypertension at baseline and delta BMI and years with CPAP during follow-up (106).
Mortality
Clinic-based studies suggest that patients with OSA syndrome have a higher mortality risk (107) and that treatment with tracheostomy or CPAP attenuates this risk (108-110). The lack of randomized, controlled interventional trials clearly limits the evidence, as non-treated patients have either been non-compliant with prescribed therapy or have for some reason not been selected for effective treatment. Clinical mortality studies might also be biased, as patients under treatment for some other serious morbidity might also be more likely to be referred for an evaluation of sleep apnea, leading to an overestimation of mortality.
The results diverge in studies investigating whether patients with OSA syndrome have a shorter survival or not. No increased mortality rate was found between apnea-hypopnea scores in two prospective studies investigating elderly populations (111,112), while a significant association was seen in another study, but in women only (113). Lavie et al. in a prospective study found that the apnea index was a predictor of excess mortality in the fourth and fifth decade but not in elderly men (107). This is in accordance with the results from of a population-based study from Uppsala in Sweden where men aged 30-69 years were investigated by postal questionnaire and followed over 10 years (114). Snoring men reporting excess daytime sleepiness had a significant increase in mortality, but the age-adjusted relative risk decreased with increasing age and was no longer significant after age 50 years. Snoring alone had no impact on mortality in any of the age groups (Table 2).

Full table
The impact of OSA on mortality in population-based cohorts has recently been analyzed in the Wisconsin Study (23), and in the Sleep Heart Health Study (115), and both reported an increased mortality rate with increasing severity of sleep apnea (Table 2). Subjects with an AHI ≥30 had an adjusted hazard ratio for all-cause mortality of 3.0 (95% CI, 1.4-6.3) and 1.46 (95% CI, 1.14-1.86) respectively, compared with those with an AHI of <5. Similar results were obtained for cardiovascular mortality in both studies and the exclusion of subjects treated for sleep apnea did not change the results. However, the adjusted hazard ratios for severe sleep apnea only remained significant in younger men <70 years in the Sleep Heart Health Study.
Conclusions
OSA is highly prevalent in the population. It is related to age and obesity. Only a part of subjects with OSA in the population have symptoms in the form of daytime sleepiness. The prevalence of OSA and OSA syndrome has increased in epidemiological studies over time. Differences and the increase in prevalence of sleep apnea are probably due to different diagnostic equipment, definitions, study design and characteristics of included subjects. Cardiovascular disease, especially stroke is related to OSA and subjects under the age of 70 run an increased risk of early death if they suffer from OSA.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors declare no conflict of interest.
References
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667-89. [PubMed]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5. [PubMed]
- Franklin KA, Sahlin C, Stenlund H, et al. Sleep apnoea is a common occurrence in females. Eur Respir J 2013;41:610-5. [PubMed]
- Lavie P. Incidence of sleep apnea in a presumably healthy working population: a significant relationship with excessive daytime sleepiness. Sleep 1983;6:312-8. [PubMed]
- Gislason T, Almqvist M, Eriksson G, et al. Prevalence of sleep apnea syndrome among Swedish men--an epidemiological study. J Clin Epidemiol 1988;41:571-6. [PubMed]
- Cirignotta F, D’Alessandro R, Partinen M, et al. Prevalence of every night snoring and obstructive sleep apnoeas among 30-69-year-old men in Bologna, Italy. Acta Neurol Scand 1989;79:366-72. [PubMed]
- Schmidt-Nowara JR, Jennum P. Epidemiology of sleep apnea. In: Guilleminault C, Partinen M, editors. Obstructive sleep apnea syndrome—clinical research and treatment. New York: Raven Press, 1990:1-8.
- Bixler EO, Vgontzas AN, Ten Have T, et al. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med 1998;157:144-8. [PubMed]
- Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5. [PubMed]
- Durán J, Esnaola S, Rubio R, et al. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med 2001;163:685-9. [PubMed]
- Ip MS, Lam B, Tang LC, et al. A community study of sleep-disordered breathing in middle-aged Chinese women in Hong Kong: prevalence and gender differences. Chest 2004;125:127-34. [PubMed]
- Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest 2001;119:62-9. [PubMed]
- Udwadia ZF, Doshi AV, Lonkar SG, et al. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian men. Am J Respir Crit Care Med 2004;169:168-73. [PubMed]
- Kim J, In K, Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med 2004;170:1108-13. [PubMed]
- Sharma SK, Kumpawat S, Banga A, et al. Prevalence and risk factors of obstructive sleep apnea syndrome in a population of Delhi, India. Chest 2006;130:149-56. [PubMed]
- Nakayama-Ashida Y, Takegami M, Chin K, et al. Sleep-disordered breathing in the usual lifestyle setting as detected with home monitoring in a population of working men in Japan. Sleep 2008;31:419-25. [PubMed]
- Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 2001;163:608-13. [PubMed]
- Schmidt-Nowara WW, Coultas DB, Wiggins C, et al. Snoring in a Hispanic-American population. Risk factors and association with hypertension and other morbidity. Arch Intern Med 1990;150:597-601. [PubMed]
- Lindberg E, Janson C, Gislason T, et al. Sleep disturbances in a young adult population: can gender differences be explained by differences in psychological status? Sleep 1997;20:381-7. [PubMed]
- Nagayoshi M, Yamagishi K, Tanigawa T, et al. Risk factors for snoring among Japanese men and women: a community-based cross-sectional study. Sleep Breath 2011;15:63-9. [PubMed]
- Guilleminault C, Quera-Salva MA, Partinen M, et al. Women and the obstructive sleep apnea syndrome. Chest 1988;93:104-9. [PubMed]
- Quintana-Gallego E, Carmona-Bernal C, Capote F, et al. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med 2004;98:984-9. [PubMed]
- Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008;31:1071-8. [PubMed]
- Hader C, Schroeder A, Hinz M, et al. Sleep disordered breathing in the elderly: comparison of women and men. J Physiol Pharmacol 2005;56 Suppl 4:85-91. [PubMed]
- Lindberg E, Taube A, Janson C, et al. A 10-year follow-up of snoring in men. Chest 1998;114:1048-55. [PubMed]
- Svensson M, Lindberg E, Naessen T, et al. Risk factors associated with snoring in women with special emphasis on body mass index: a population-based study. Chest 2006;129:933-41. [PubMed]
- Lindberg E, Elmasry A, Gislason T, et al. Evolution of sleep apnea syndrome in sleepy snorers: a population-based prospective study. Am J Respir Crit Care Med 1999;159:2024-7. [PubMed]
- Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000;284:3015-21. [PubMed]
- Launois SH, Pépin JL, Lévy P. Sleep apnea in the elderly: a specific entity? Sleep Med Rev 2007;11:87-97. [PubMed]
- Bearpark H, Elliott L, Grunstein R, et al. Snoring and sleep apnea. A population study in Australian men. Am J Respir Crit Care Med 1995;151:1459-65. [PubMed]
- Koskenvuo M, Partinen M, Kaprio J, et al. Snoring and cardiovascular risk factors. Ann Med 1994;26:371-6. [PubMed]
- Strohl KP, Redline S. Recognition of obstructive sleep apnea. Am J Respir Crit Care Med 1996;154:279-89. [PubMed]
- Barvaux VA, Aubert G, Rodenstein DO. Weight loss as a treatment for obstructive sleep apnoea. Sleep Med Rev 2000;4:435-52. [PubMed]
- Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol (1985) 2005;99:1592-9. [PubMed]
- Grunstein RR, Stenlöf K, Hedner JA, et al. Two year reduction in sleep apnea symptoms and associated diabetes incidence after weight loss in severe obesity. Sleep 2007;30:703-10. [PubMed]
- Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med 2009;122:535-42. [PubMed]
- Johansson K, Hemmingsson E, Harlid R, et al. Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised controlled trial: prospective observational follow-up study. BMJ 2011;342:d3017. [PubMed]
- Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med 2014;370:2265-75. [PubMed]
- Newman AB, Foster G, Givelber R, et al. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med 2005;165:2408-13. [PubMed]
- Shelton KE, Woodson H, Gay S, et al. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis 1993;148:462-6. [PubMed]
- Lecube A, Sampol G, Lloberes P, et al. Asymptomatic sleep-disordered breathing in premenopausal women awaiting bariatric surgery. Obes Surg 2010;20:454-61. [PubMed]
- Jennum P, Sjøl A. Epidemiology of snoring and obstructive sleep apnoea in a Danish population, age 30-60. J Sleep Res 1992;1:240-4. [PubMed]
- Hu FB, Willett WC, Colditz GA, et al. Prospective study of snoring and risk of hypertension in women. Am J Epidemiol 1999;150:806-16. [PubMed]
- Franklin KA, Gíslason T, Omenaas E, et al. The influence of active and passive smoking on habitual snoring. Am J Respir Crit Care Med 2004;170:799-803. [PubMed]
- Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax 1991;46:85-90. [PubMed]
- Wetter DW, Young TB, Bidwell TR, et al. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med 1994;154:2219-24. [PubMed]
- Pack AI, Cola MF, Goldszmidt A, et al. Correlation between oscillations in ventilation and frequency content of the electroencephalogram. J Appl Physiol (1985) 1992;72:985-92. [PubMed]
- Newman AB, Nieto FJ, Guidry U, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol 2001;154:50-9. [PubMed]
- Krol RC, Knuth SL, Bartlett D Jr. Selective reduction of genioglossal muscle activity by alcohol in normal human subjects. Am Rev Respir Dis 1984;129:247-50. [PubMed]
- Issa FG, Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry 1982;45:353-9. [PubMed]
- Dolly FR, Block AJ. Increased ventricular ectopy and sleep apnea following ethanol ingestion in COPD patients. Chest 1983;83:469-72. [PubMed]
- Jennum P, Sjøl A. Snoring, sleep apnoea and cardiovascular risk factors: the MONICA II Study. Int J Epidemiol 1993;22:439-44. [PubMed]
- Peppard PE, Austin D, Brown RL. Association of alcohol consumption and sleep disordered breathing in men and women. J Clin Sleep Med 2007;3:265-70. [PubMed]
- Worsnop CJ, Naughton MT, Barter CE, et al. The prevalence of obstructive sleep apnea in hypertensives. Am J Respir Crit Care Med 1998;157:111-5. [PubMed]
- Engleman HM, Kingshott RN, Wraith PK, et al. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep Apnea/Hypopnea syndrome. Am J Respir Crit Care Med 1999;159:461-7. [PubMed]
- Ballester E, Badia JR, Hernández L, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 1999;159:495-501. [PubMed]
- Jenkinson C, Davies RJ, Mullins R, et al. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet 1999;353:2100-5. [PubMed]
- Faccenda JF, Mackay TW, Boon NA, et al. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med 2001;163:344-8. [PubMed]
- Patel SR, White DP, Malhotra A, et al. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003;163:565-71. [PubMed]
- McDaid C, Durée KH, Griffin SC, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea-hypopnoea syndrome. Sleep Med Rev 2009;13:427-36. [PubMed]
- Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med 1999;159:502-7. [PubMed]
- Theorell-Haglöw J, Lindberg E, Janson C. What are the important risk factors for daytime sleepiness and fatigue in women? Sleep 2006;29:751-7. [PubMed]
- Svensson M, Franklin KA, Theorell-Haglöw J, et al. Daytime sleepiness relates to snoring independent of the apnea-hypopnea index in women from the general population. Chest 2008;134:919-24. [PubMed]
- Stradling JR, Barbour C, Glennon J, et al. Prevalence of sleepiness and its relation to autonomic evidence of arousals and increased inspiratory effort in a community based population of men and women. J Sleep Res 2000;9:381-8. [PubMed]
- Kapur VK, Baldwin CM, Resnick HE, et al. Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep 2005;28:472-7. [PubMed]
- Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med 2006;166:1716-22. [PubMed]
- Walsleben JA, Kapur VK, Newman AB, et al. Sleep and reported daytime sleepiness in normal subjects: the Sleep Heart Health Study. Sleep 2004;27:293-8. [PubMed]
- Hanly P. Sleep disorders and end-stage renal disease. Curr Opin Pulm Med 2008;14:543-50. [PubMed]
- Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med 1997;157:1746-52. [PubMed]
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000;283:1829-36. [PubMed]
- Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med 2000;160:2289-95. [PubMed]
- Haas DC, Foster GL, Nieto FJ, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation 2005;111:614-21. [PubMed]
- Lindberg E, Janson C, Gislason T, et al. Snoring and hypertension: a 10 year follow-up. Eur Respir J 1998;11:884-9. [PubMed]
- Kim J, Yi H, Shin KR, et al. Snoring as an independent risk factor for hypertension in the nonobese population: the Korean Health and Genome Study. Am J Hypertens 2007;20:819-24. [PubMed]
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378-84. [PubMed]
- Hla KM, Young T, Finn L, et al. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep 2008;31:795-800. [PubMed]
- Sjöström C, Lindberg E, Elmasry A, et al. Prevalence of sleep apnoea and snoring in hypertensive men: a population based study. Thorax 2002;57:602-7. [PubMed]
- Lindberg E, Berne C, Franklin KA, et al. Snoring and daytime sleepiness as risk factors for hypertension and diabetes in women--a population-based study. Respir Med 2007;101:1283-90. [PubMed]
- Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med 2007;167:757-64. [PubMed]
- D'Alessandro R, Magelli C, Gamberini G, et al. Snoring every night as a risk factor for myocardial infarction: a case-control study. BMJ 1990;300:1557-8. [PubMed]
- Franklin KA, Nilsson JB, Sahlin C, et al. Sleep apnoea and nocturnal angina. Lancet 1995;345:1085-7. [PubMed]
- Mooe T, Rabben T, Wiklund U, et al. Sleep-disordered breathing in men with coronary artery disease. Chest 1996;109:659-63. [PubMed]
- Olson LG, King MT, Hensley MJ, et al. A community study of snoring and sleep-disordered breathing. Health outcomes. Am J Respir Crit Care Med 1995;152:717-20. [PubMed]
- Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001;163:19-25. [PubMed]
- Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J 2006;28:596-602. [PubMed]
- Buchner NJ, Sanner BM, Borgel J, et al. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med 2007;176:1274-80. [PubMed]
- Milleron O, Pillière R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J 2004;25:728-34. [PubMed]
- Spriggs DA, French JM, Murdy JM, et al. Effect of the risk factors for stroke on survival. Neurol Res 1992;14:94-6. [PubMed]
- Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034-41. [PubMed]
- Valham F, Mooe T, Rabben T, et al. Increased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-up. Circulation 2008;118:955-60. [PubMed]
- Sahlin C, Sandberg O, Gustafson Y, et al. Obstructive sleep apnea is a risk factor for death in patients with stroke: a 10-year follow-up. Arch Intern Med 2008;168:297-301. [PubMed]
- Munoz R, Duran-Cantolla J, Martínez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke 2006;37:2317-21. [PubMed]
- Arzt M, Young T, Finn L, et al. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med 2005;172:1447-51. [PubMed]
- Shin C, Kim J, Kim J, et al. Association of habitual snoring with glucose and insulin metabolism in nonobese Korean adult men. Am J Respir Crit Care Med 2005;171:287-91. [PubMed]
- Joo S, Lee S, Choi HA, et al. Habitual snoring is associated with elevated hemoglobin A1c levels in non-obese middle-aged adults. J Sleep Res 2006;15:437-44. [PubMed]
- Elmasry A, Lindberg E, Berne C, et al. Sleep-disordered breathing and glucose metabolism in hypertensive men: a population-based study. J Intern Med 2001;249:153-61. [PubMed]
- Ip MS, Lam B, Ng MM, et al. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med 2002;165:670-6. [PubMed]
- Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004;160:521-30. [PubMed]
- Reichmuth KJ, Austin D, Skatrud JB, et al. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med 2005;172:1590-5. [PubMed]
- Theorell-Haglöw J, Berne C, Janson C, et al. Obstructive sleep apnoea is associated with decreased insulin sensitivity in females. Eur Respir J 2008;31:1054-60. [PubMed]
- Punjabi NM, Beamer BA. Alterations in Glucose Disposal in Sleep-disordered Breathing. Am J Respir Crit Care Med 2009;179:235-40. [PubMed]
- Elmasry A, Janson C, Lindberg E, et al. The role of habitual snoring and obesity in the development of diabetes: a 10-year follow-up study in a male population. J Intern Med 2000;248:13-20. [PubMed]
- Al-Delaimy WK, Manson JE, Willett WC, et al. Snoring as a risk factor for type II diabetes mellitus: a prospective study. Am J Epidemiol 2002;155:387-93. [PubMed]
- Marshall NS, Wong KK, Phillips CL, et al. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med 2009;5:15-20. [PubMed]
- Botros N, Concato J, Mohsenin V, et al. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med 2009;122:1122-7. [PubMed]
- Lindberg E, Theorell-Haglöw J, Svensson M, et al. Sleep apnea and glucose metabolism: a long-term follow-up in a community-based sample. Chest 2012;142:935-42. [PubMed]
- Lavie P, Herer P, Peled R, et al. Mortality in sleep apnea patients: a multivariate analysis of risk factors. Sleep 1995;18:149-57. [PubMed]
- He J, Kryger MH, Zorick FJ, et al. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest 1988;94:9-14. [PubMed]
- Partinen M, Jamieson A, Guilleminault C. Long-term outcome for obstructive sleep apnea syndrome patients. Mortality. Chest 1988;94:1200-4. [PubMed]
- Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53. [PubMed]
- Bliwise DL, Bliwise NG, Partinen M, et al. Sleep apnea and mortality in an aged cohort. Am J Public Health 1988;78:544-7. [PubMed]
- Mant A, King M, Saunders NA, et al. Four-year follow-up of mortality and sleep-related respiratory disturbance in non-demented seniors. Sleep 1995;18:433-8. [PubMed]
- Ancoli-Israel S, Klauber MR, Kripke DF, et al. Sleep apnea in female patients in a nursing home. Increased risk of mortality. Chest 1989;96:1054-8. [PubMed]
- Lindberg E, Janson C, Svärdsudd K, et al. Increased mortality among sleepy snorers: a prospective population based study. Thorax 1998;53:631-7. [PubMed]
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009;6:e1000132. [PubMed]


