Challenges in valve-in-valve therapy
Challenges in valve-in-valve (VIV) therapy
Although bioprosthetic valves are less thrombogenic than their mechanical counterparts, they have limited durability and experience structural deterioration over time. This process usually starts 10-15 years following the initial surgical implantation and usually by this stage, up to 10-30% of valves show evidence of deterioration. This number rises up to 30-60% at 15 years following implantation (1,2).
The VIV procedure comprises of percutaneous implantation of a transcatheter heart valve (THV) within an existing degenerated surgical heart valve (SHV), in a suitable patient and is a natural evolution of the transcatheter aortic valve implantation (TAVI) procedure (1-3). It follows therefore, that the operator must have a detailed understanding of:
- The anatomy and fluoroscopic appearances of SHV, as not all SHVs are the same;
- THV designs as not all THVs are the same;
- The correct sizing of the chosen THV for the existing SHV;
- The ideal implantation position for the chosen THV within the existing SHV.
Stented SHV design
A detailed understanding in the variation of SHV design is important to allow the optimal selection of a suitable THV for a given SHV. This will aid in reducing potential complications such as THV misplacement, embolization and coronary obstruction.
SHVs can be broadly classified as stented or stentless, based on the presence or absence of a rigid pericardium or fabric covered stent frame. Stented valves consist of a rigid frame and three struts or posts within which three bovine pericardial or porcine leaflets are suspended. A fabric covered sewing ring forms the base of the valve and this is sewn onto the native valve annulus at surgery (4) (Figure 1).
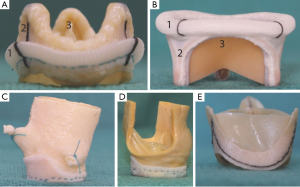
Stented valves may be further sub-classified depending on:
- The type and arrangement of leaflets with respect to the stent frame;
- Their fluoroscopic appearance;
- Their function after implantation—supra or intra-annular.
These are discussed below.
- The type and arrangement of the leaflets with respect to the stent frame, for an aortic SHV could be:
- Porcine leaflets placed within the stent frame;
- Bovine pericardial leaflets sutured inside the frame or;
- Bovine pericardial leaflets sutured outside the stent frame.
- Fluoroscopic appearance: SHVs can have a visible or radio-opaque sewing ring, stent frame or none of the components may be visible, i.e., not radio-opaque.
- The intended function of the SHV following surgical implantation, in the case of aortic valves, as these can be supra-annular or intra-annular.
The type and arrangement of leaflets governs the ‘true internal diameter (ID)’ of the SHV. This is an important concept as it can affect the choice of THV implant size and type and is discussed further below (5). Stented mitral SHVs do not have leaflets positioned outside the frame.
Stentless SHV design
Stentless valves by definition do not possess a rigid stent frame and they can be surgically implanted in one of two ways (6); (I) subcoronary placement; (II) full root replacement.
It is essential to confirm the nature of the original surgical implantation as different implantation techniques may be subject to different challenges when considering a VIV procedure. In the subcoronary technique, the suture line between the stentless prosthesis and the aorta is close to the native coronary ostia (6) (Figure 2).
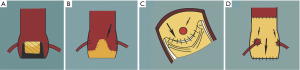
There is a potential higher risk of coronary artery obstruction in these cases, as it is feasible that during a VIV, the SHV leaflets are pushed outwards and thereby blocking the ostia. Obstruction is less of a possibility during a full root replacement as the finished result resembles a native aortic root, within which performing a VIV should be essentially the same as performing one in a native aorta (6) (Figure 2).
THV design
As with any novel therapy, there has been considerable development in the design and types of THVs available in the medical market. The two most commonly used THVs for the VIV procedure are the Edwards Sapien valve (ESV), (Edwards Lifesciences, Irvine, CA, USA), which has been used in all four valve positions, and the Medtronic CoreValve (MCV) (Medtronic Inc., Minneapolis, MN, USA) used in the aortic position alone. Other more recent additions to the market but used in the aortic position alone are the St. Jude Portico valve (SPV), (St. Jude Medical, St. Paul, MN, USA), the JenaValve (JV) (JenaValve, Munich, Germany), and the Symetis valve (SV) (Symetis, Vaud, Switzerland). The Melody valve (Medtronic Inc., Minneapolis, MN, USA) has been used in both the pulmonary and mitral positions but experience is limited (7-9) (Figure 3).

The operator must be familiar with the differences in THV design as these may dictate their best position during a VIV procedure as well as with ID ranges for which they are suitable as these are different for each THV type (Table 1).
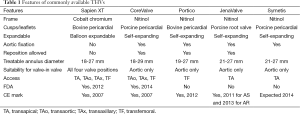
Full table
Correct sizing to ensure an ideal match between the SHV and THV
Manufacturers of SHVs often label their valves according to the outer stent diameter and there is therefore considerable variation in the stent ID between SHVs of same label size (10,11) (Table 2). Additionally, the stent ID may not correctly reflect the ID as the type and arrangement of leaflets can significantly reduce the stent ID of the SHV and will impact choice of the THV (5) (Figure 4). For this reason, it is imperative that the operator be aware of these design implications when selecting a suitable THV. Table 2 shows the differences in dimensions between a labeled size 27 SHVs from different manufacturers. To ensure a successful outcome during a VIV procedure the true ID of a stented valve should be used to select an appropriate THV rather than the valve label size or the stent ID (5).

Full table
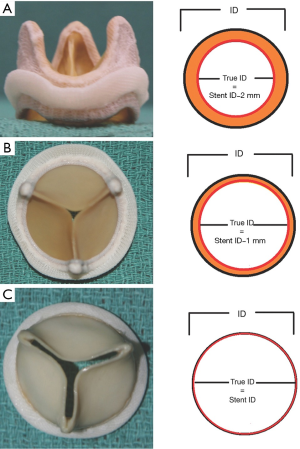
In the case of stentless valves, the size of the THV used should be according to either the tissue annulus diameter of the stentless root or echocardiographic or mutli-slice CT measurements of the aortic root similar to the native aortic root (6).
Ideal implant position of THV during the VIV procedure
It is essential to ensure that the implant position of a THV within a given SHV is in its ideal position as too low a position can lead to suboptimal function or paravalvular regurgitation and too high a position may lead to coronary obstruction or embolization (12).
During a TAVI, the level of the aortic annulus is taken as a reference level for THV implantation. Thus, an ESV is implanted no more than 50% below the annulus, MCV is implanted 4 mm below the annulus and SPV is implanted 5 mm below the annulus. During a VIV procedure, the level of the sewing ring of the SHV i.e., the neo-annulus should be used as a reference plane (13).
Identification of this neo-annulus plane, depending on what part of the SHV is fluoroscopically visible and whether it is supra or intra annular in design is critical. Thus, when the sewing ring of the SHV is fluoroscopically visible, it should be used as a reference plane for positioning of the intended THV. When the stent frame is visible, the level of the sewing ring varies depending on whether the SHV is supra or intra-annular design. It is important to understand this difference (12). If none of the SHV components are visible example in an Intact SHV (Medtronic Inc., Minneapolis, MN, USA) positioning under echocardiography should be used during a VIV procedure. The Mosaic SHV (Medtronic Inc. Minneapolis, Minnesota, USA) is unique in its features as only the stent frame tips are visible (Figure 5). To avoid malposition, one can use these post tips as a guide to deploy THV depending on what type is used (12) (Figure 6).

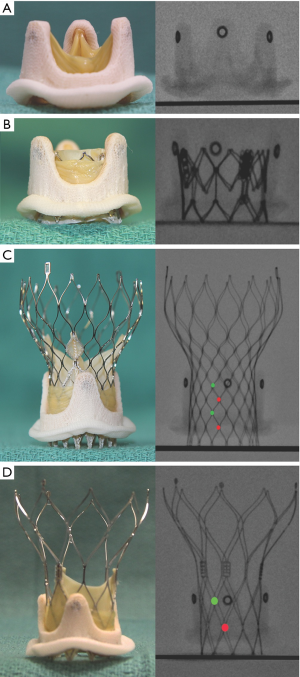
Stentless valves present a unique challenge, as they are radiolucent with no identifiable fluoroscopic features. Implantation of a THV within these has to be guided by echocardiography or multiple contrast injections to delineate the level of the neo-annulus. Further, slow deployment is critical for accurate placement (6).
Potential challenges and complications
The failure to accurately size, position and implant a suitable THV within an existing SHV can lead to procedural failure and poor patient outcome.
Specific challenges pertaining to incorrect sizing and placement of a THV within an SHV include:
- Coronary obstruction especially in the case of stentless valves;
- THV migration and embolization which may be immediate or delayed;
- High residual gradients.
Coronary obstruction
Coronary obstruction, particularly of the left coronary artery is now a well-documented complication, although it was not initially readily anticipated, given that THVs were expected to remain within the rigid SHV frame. The Valve in Valve International Data Registry (VIVIDR) or Global VIV Registry has reported a frequency of coronary artery obstruction of 3.5%, which is higher than the incidence of coronary obstruction from a native valve procedure (15,16).
A common mode of obstruction is due to the bioprosthetic leaflets being pushed outwards by the THV, thereby coming into direct contact with the coronary ostia or the sinotubular junction overlying the ostia. This potential complication can occur with any of the THVs available, but more so if the aortic root is small and the THV is grossly oversized compared to the true ID of the SHV. For example, when a 23 ESV is deployed within a 23 Mitroflow (true ID of 19 mm) the stent posts will be pushed out by at least 4 mm. Use of the recently available 20 ESV in this case would be ideal.
Other factors, which should also raise the possibility of potential coronary obstruction are (2,16):
- Low lying coronary ostia;
- Specific SHV design with leaflets outside the stent frame (trifecta, mitroflow);
- Bulky bioprosthetic valve leaflets;
- Stentless valves;
- High implantation of a THV.
If coronary obstruction is anticipated then either a balloon aortic valvuloplasty (BAV) using an appropriately sized balloon with contrast injection prior to THV deployment, or guarding of the coronary ostia with wires is advisable. If coronary obstruction is detected after the VIV implantation, immediate access to coronary arteries must be gained and a stent placed to reopen the coronary (16). If needed cardio-pulmonary bypass should be initiated with peripheral cannulation so as to stabilize the situation while gaining access to the coronaries. With the availability of second-generation retrievable and repositionable devices such as the Evolut R (Medtronic Inc. Minneapolis, MN, USA), Portico (St Jude Medical, St. Paul, MN) and Lotus (Boston Scientific Corp. Marlborough, MA, USA), the incidence of coronary obstruction should be seen to decline.
Migration and embolization
Intra-procedural migration and embolization of both the MCV and ESV during an aortic VIV procedure, due to suboptimal sizing (smaller THV) and positioning (too low a placement) have been reported in the literature. In fact, initial results from the VIVIDR registry reported malpositioning in some 15% of cases resulting in the need for additional procedures, including placement of an additional THV in 8.4% cases, attempted retrieval in 8.9% and balloon valvuloplasty post implantation in 12.4% cases (15). This is a result of either using a smaller size THV or improper placement. Attention to choosing appropriate size THV by using the true ID of the SHV as a guide and ideal placement is essential to avoid this complication. Slow deployment or a two-stage deployment for the ESV will also reduce this complication. Additionally, repositionability will be an important design modification for self-expanding THVs allowing confirmation of an ideal position before deployment.
High residual gradients
High residual gradients are an Achilles heel of aortic VIV procedures. The VIVIDR reported an incidence of high gradients (mean gradient >20 mmHg) in 28% of cases and these were predominantly in the ESV rather than the MCV group. Additionally, there was a significant difference in the rate of higher post-procedural gradients between the ESV (58%) vs. MCV (20%) for VIV performed in smaller SHVs (<20 mm in ID) (15).
Overall, the aetiology of high post-procedural gradients is multifactorial. Two important factors are the true ID of the SHVs being treated and the available sizes of THVs. Thus, if treating SHVs with labeled sizes ranging between 19 or 21 mm, the true ID is actually less than 19 mm. The smallest THV till recently available and used was a 23 mm ESV or MCV. It is therefore obvious that there will be incomplete expansion of the THV due to a size mismatch resulting in higher residual gradients. Therefore patients with smaller sizes of SHVs should not be considered for VIV therapy, if redo-surgery is feasible.
It has been suggested that the MCV, due to its supra-annular structure leads to lower residual gradients than the ESV when compared for similar SHV stent ID treated and should be preferentially used for this indication (15). The data however need to be re-examined by using the true ID of SHVs as we know that the same stent ID does not equate to same true ID in SHVs.
Mitral VIV
Currently only the ESV can be used to perform a VIV in the mitral position. Mitral SHVs are similar to aortic SHVs in structure and design but the size range is usually larger (25 to 29 or 33 mm). Thus, in the case of a mitral VIV procedure, a larger ESV can be used in majority of the cases. There is however a possibility that the largest size mitral SHVs may not be suitable for the largest ESV available i.e., 29 mm.
Although coronary obstruction is not a problem with mitral VIV, it is associated with a unique problem i.e., delayed embolization (17). The potential explanations for this could be the higher closing pressure on the mitral valve, which is systolic compared to the closing pressure on the aortic valve, which is diastolic. To avoid this problem, a larger oversizing to achieve a flare at the ventricular end may be essential (17).
Mitral valve-in-ring
Mitral valve-in-ring procedures are also being performed more frequently. These deserve special consideration, as no two rings are similar in shape, size, rigidity and their fluoroscopic properties.
From the limited experience in this procedure it appears that flexible and semi-flexible rings such as Duran Ancore (Medtronic Inc. Minneapolis, MN, USA) and Physio 1 (Edwards Lifesciences, Irvine, CA, USA) respectively are suitable for a valve-in-ring procedure, provided their size fits within the available ESV range (18). Rigid rings may not be suited to a valve-in-ring procedure, as they cannot be deformed in to a circular shape and incomplete rings may prevent complete deployment of the THV leading to paravaluvular leaks. Similarly, certain rings for example the Seguin (St Jude Medical, St. Paul, MN, USA) are not radio-opaque will pose different challenges during this procedure.
Other considerations
In the case of double VIV procedures (aortic and mitral), where trans-apical access is preferred by most operators, a careful consideration must be given to the sequence of placement of the respective valves. The aortic THV must be placed first followed by the mitral for technical ease (19). This is because if the mitral VIV is done first, it may become difficult to pass the THV in to the aortic position.
VIV in the tricuspid and pulmonary positions are also being performed. At present only the ESV (Sapien XT) is suitable for these cases and can be implanted through the trans-atrial, trans-jugular and trans-femoral venous routes using either the trans-apical or trans-femoral delivery systems depending on ease and operator preference. through trans-atrial, trans-jugular and trans-femoral venous access using either the transapical or transfemoral delivery systems depending on ease and operator preference (8,9,20,21).
Conclusions
VIV therapy is a rapidly growing alternative to a redo operation in high-risk patients when treating degenerated SHVs. However it should not be used indiscriminately especially when the SHV size is small, as early results have demonstrated unique problems. Increasing experience and mid-term results will define the future of this therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: Mr. Vinayak Bapat is a consultant for Edwards Lifesciences, Medtronic Inc. and Boston Scientific. Miss Alia Noorani and Miss Rahee Radia have no conflicts of interest to declare.
References
- Webb JG, Dvir D. Transcatheter aortic valve replacement for bioprosthetic aortic valve failure: the valve-in-valve procedure. Circulation 2013;127:2542-50. [PubMed]
- Dvir D, Barbanti M, Tan J, et al. Transcatheter aortic valve-in-valve implantation for patients with degenerative surgical bioprosthetic valves. Curr Probl Cardiol 2014;39:7-27. [PubMed]
- Svensson LG, Tuzcu M, Kapadia S, et al. A comprehensive review of the PARTNER trial. J Thorac Cardiovasc Surg 2013;145:S11-6. [PubMed]
- Bapat V, Mydin I, Chadalavada S, et al. A guide to fluoroscopic identification and design of bioprosthetic valves: a reference for valve-in-valve procedure. Catheter Cardiovasc Interv 2013;81:853-61. [PubMed]
- Bapat VN, Attia R, Thomas M. Effect of valve design on the stent internal diameter of a bioprosthetic valve: a concept of true internal diameter and its implications for the valve-in-valve procedure. JACC Cardiovasc Interv 2014;7:115-27. [PubMed]
- Bapat V, Davies W, Attia R, et al. Use of balloon expandable transcatheter valves for valve-in-valve implantation in patients with degenerative stentless aortic bioprostheses: Technical considerations and results. J Thorac Cardiovasc Surg 2014;148:917-22; discussion 922-4. [PubMed]
- Cullen MW, Cabalka AK, Alli OO, et al. Transvenous, antegrade Melody valve-in-valve implantation for bioprosthetic mitral and tricuspid valve dysfunction: a case series in children and adults. JACC Cardiovasc Interv 2013;6:598-605. [PubMed]
- Roberts PA, Boudjemline Y, Cheatham JP, et al. Percutaneous tricuspid valve replacement in congenital and acquired heart disease. J Am Coll Cardiol 2011;58:117-22. [PubMed]
- Tzifa A, Momenah T, Al Sahari A, et al. Transcatheter valve-in-valve implantation in the tricuspid position. EuroIntervention 2014;10:995-9. [PubMed]
- Christakis GT, Buth KJ, Goldman BS, et al. Inaccurate and misleading valve sizing: a proposed standard for valve size nomenclature. Ann Thorac Surg 1998;66:1198-203. [PubMed]
- Doenst T, Amorim PA, Al-Alam N, et al. Where is the common sense in aortic valve replacement? A review of hemodynamics and sizing of stented tissue valves. J Thorac Cardiovasc Surg 2011;142:1180-7. [PubMed]
- Bapat VN, Attia RQ, Condemi F, et al. Fluoroscopic guide to an ideal implant position for Sapien XT and CoreValve during a valve-in-valve procedure. JACC Cardiovasc Interv 2013;6:1186-94. [PubMed]
- Bapat V, Adams B, Attia R, et al. Neo-annulus: a reference plane in a surgical heart valve to facilitate a valve-in-valve procedure. Catheter Cardiovasc Interv 2015;85:685-91. [PubMed]
- Noorani A, Attia R, Bapat V. Valve-in-valve procedure: importance of the anatomy of surgical bioprostheses. Multimed Man Cardiothorac Surg 2014;2014.
- Dvir D, Webb J, Brecker S, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation 2012;126:2335-44. [PubMed]
- Ribeiro HB, Webb JG, Makkar RR, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol 2013;62:1552-62. [PubMed]
- Bapat VV, Khaliel F, Ihleberg L. Delayed migration of Sapien valve following a transcatheter mitral valve-in-valve implantation. Catheter Cardiovasc Interv 2014;83:E150-4. [PubMed]
- Descoutures F, Himbert D, Maisano F, et al. Transcatheter valve-in-ring implantation after failure of surgical mitral repair. Eur J Cardiothorac Surg 2013;44:e8-15. [PubMed]
- D’Onofrio A, Zucchetta F, Gerosa G. Simultaneous transapical aortic and mitral valve-in-valve implantation for double prostheses dysfunction: case report and technical insights. Catheter Cardiovasc Interv 2014;84:509-12. [PubMed]
- McElhinney DB, Hellenbrand WE, Zahn EM, et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation 2010;122:507-16. [PubMed]
- Khambadkone S, Coats L, Taylor A, et al. Percutaneous pulmonary valve implantation in humans: results in 59 consecutive patients. Circulation 2005;112:1189-97. [PubMed]


