Spontaneous pneumothorax as the first manifestation of lung cancer: two case report
Introduction
Spontaneous pneumothorax (SP) can be divided into two types, primary and secondary, according to the presence of underlying pulmonary pathology (1-3). Different from the primary SP with subpleural bullous dystrophy only, secondary SP develops with various pulmonary conditions, including obstruction, infection, infarction, neoplasm and diffuse lung disease (4-10).
In this paper we report two cases of lung cancer, in which the initial manifestations was only limited to SP.
Case report
Case 1
A 70-year-old man was admitted to our hospital with chest wall pain and dyspnea. He did not have any history of previous trauma, operation or malignancy. Physical examination showed markedly decreased breathing sounds in the right side of the chest. There were no abnormal findings on laboratory tests. Chest X-ray showed right-sided pneumothorax with total collapsed right-side lung (Figure 1).
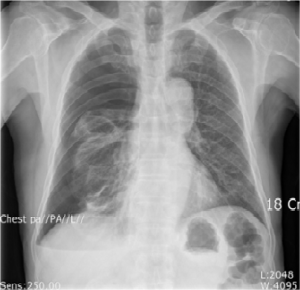
A 28-Fr chest tube was inserted, and computed tomography (CT) of the chest was performed. The CT revealed a right sided pneumothorax and several bullae on the right upper lobe (RUL) of lung. No additional sign of combined pulmonary pathology was noted. With persistent air leak over 1 week, we decided to perform a wedge resection of the lung via video-assisted thoracic surgery (VATS).
An elective right sided wedge resection, via VATS, was performed. Patient was positioned laterally after intubation, using a double lumen tube. With two working ports and one camera port, wedge resection for multiple small sized bullae at the RUL was performed in conventional manner. Thoracic cavity was thoroughly investigated for combined pathologic change, with no result. The specimen was sent for a pathologic study. The patient was stable throughout the operation, and was sent to the recovery room.
The postoperative recovery was uneventful, except mild air leak through the chest tube which resolved 1 week later. The specimen, sent for pathologic evaluation revealed high grade mucoepidermoid carcinoma of the lung invading the adjacent arterial wall. With the final pathologic staging confirmed as pT1aN0M0, VATS RULobectomy was performed in the conventional manner.
Case 2
A 41-year-old man, with previous history of recurrent pneumothorax, was admitted to our hospital with chest wall pain and dyspnea. Except for the history of pneumothorax and wedge resection for pneumothorax (5 months prior to this admission), he had no history of previous trauma or malignancy. A physical examination showed markedly decreased breathing sounds in the left side of the chest. There were no abnormal findings on laboratory tests. On chest X-ray, total collapse of the left lung with tracheal deviation was revealed.
A 28-Fr chest tube was inserted, and CT of the chest/abdomen was performed. The chest CT revealed left sided pneumothorax and several bullae on the left upper lobe (LUL) (Figure 2). No additional sign of combined pulmonary pathology was noted. Due to the frequent recurrence, we decided to perform wedge resection of the lung, via a VATS.
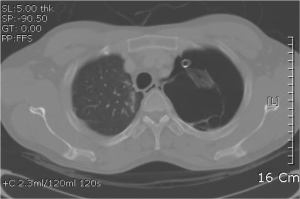
An elective left sided wedge resection via VATS was performed. Patient was positioned laterally after intubation, using a double lumen tube. Using the conventional manner, a wedge resection for multiple small sized bullae on the LUL was performed. Thoracic cavity was thoroughly investigated for combined pathologic change, with no result. The specimen was sent for pathologic study. The patient was stable throughout the operation, and was sent to the recovery room.
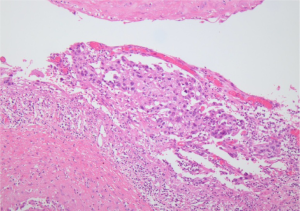
The postoperative recovery was uneventful. The specimen was sent for pathologic evaluation, and revealed non-small cell carcinoma in bulla space (Figure 3). The tumor cells showed positive against CK7, TTF-1 on immunohistochemistry. With the final pathologic staging confirmed as pT1aN0M0, VATS LU Lobectomy was performed in the conventional manner.
Discussion
SP combined with lung cancer, with estimated occurrence rate of between 0.03% and 0.05%, is a rare condition and is known to be detected in 2% of all SP (1,2). The malignancy occurring with SP, either primary or secondary, is summarized as metastatic germ cell tumor, osteogenic and soft tissue sarcoma and primary lung cancer (1).
According to the SP-presenting time, SP combined with malignancy can be categorized into two types. Most patients, approximately 75%, manifest SP as the presenting feature of lung cancer and shows relatively similar prognosis with patients without SP (2). Lesser patients, already known to have a lung neoplasm, suffer SP in the later courses of the disease (2).
With no consensus, various theories to explain the mechanism of SP in pulmonary neoplasm have been suggested. Some suggested that SP is a random event, regardless of the combined lung cancer, merely representing the condition of the lung, which has been exposed to the oncogenic substances, such as tobacco (2). Others accused pulmonary neoplasm itself as the prime cause of SP in lung cancer patients. Those neoplasm, mainly presented as a central mass, can precipitate the dilatation and rupture of alveoli, distal to the obstructed site (2,4). Pleural based peripheral tumor, with different mechanism, can also precipitate SP via the formation of broncho-pulmonary fistula by direct invasion. Tumor shrinkage, either by combined therapy or by self regression, is also known to precede SP by subpleural shrinkage (3).
In our cases, two different patients were diagnosed as SP combined with pulmonary neoplasm. The first patient was never diagnosed either SP or lung cancer before his 70’s. The latter has previous history of recurrent pneumothorax and wedge resection for that, even though no related malignant histologic diagnosis was achieved during the last operation. Even though different underlying mechanisms and conditions assumed, both patients were diagnosed with lung cancer incidentally during the management of SP.
Conclusions
Considering malignancy, as a cause of SP in advanced age without underlying lung disease, can be helpful in diagnosing combined malignancy earlier.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Steinhäuslin CA, Cuttat JF. Spontaneous pneumothorax. A complication of lung cancer? Chest 1985;88:709-13. [PubMed]
- O’Connor BM, Ziegler P, Spaulding MB. Spontaneous pneumothorax in small cell lung cancer. Chest 1992;102:628-9. [PubMed]
- Flood TA, Sekhon HS, Seely JM, et al. Spontaneous pneumothorax and lung carcinoma: should one consider synchronous malignant pleural mesothelioma? J Thorac Oncol 2009;4:770-2. [PubMed]
- Srinivas S, Varadhachary G. Spontaneous pneumothorax in malignancy: a case report and review of the literature. Ann Oncol 2000;11:887-9. [PubMed]
- Hanaoka N, Tanaka F, Otake Y, et al. Primary lung carcinoma arising from emphysematous bullae. Lung Cancer 2002;38:185-91. [PubMed]
- Sakuraba M, Murasugi M, Adachi T, et al. Squamous cell carcinoma caused by dysplasia on the bullous wall. Kyobu Geka 2004;57:533-6. [PubMed]
- Hirai S, Hamanaka Y, Mitsui N, et al. Primary lung cancer arising from the wall of a giant bulla. Ann Thorac Cardiovasc Surg 2005;11:109-13. [PubMed]
- Ogawa D, Shiota Y, Marukawa M, et al. Lung cancer associated with pulmonary bulla. case report and review of literature. Respiration 1999;66:555-8. [PubMed]
- Shin H, Oda M, Matsumoto I, et al. Lung adenocarcinoma originated from bulla wall accompanying spontaneous hemopneumothorax; report of a case. Kyobu Geka 2010;63:245-7. [PubMed]
- Ema T. Large cell carcinoma on the bullous wall detected in a specimen from a patient with spontaneous pneumothorax: report of a case. J Thorac Dis 2014;6:E234-6. [PubMed]


