Chronic hypoventilation syndromes and sleep-related hypoventilation
Introduction
Under physiological conditions, alveolar ventilation is closely adapted to metabolism. The minute ventilation is regulated according to the prevailing carbon dioxide production so that the arterial carbon dioxide partial pressure (PaCO2) is adjusted to values between 35-45 mmHg. Increases (hyperventilation) or decreases (hypoventilation) of the minute ventilation lead to excess elimination or accumulation of carbon dioxide, respectively, and destabilize the acid-base balance. However, consecutive increases of the pH value (respiratory alkalosis) or decreases (respiratory acidosis) are only measured during acute changes of ventilation. In contrast, if hyper- or hypoventilation continues for hours, the pH level is stabilized by variations of the renal bicarbonate (HCO3−) excretion (1). Therefore, chronic hypoventilation syndromes are characterized by:
- Diminishment of the minute ventilation, i.e., reductions of the tidal volume or breathing frequency;
- Elevation of the PaCO2 >45 mmHg;
- pH level in a normal range and an increase of HCO3− (metabolic compensation).
Chronic hypoventilation is also called hypercapnic respiratory failure or type II respiratory failure. The diminution of minute ventilation can generate from all levels of the respiratory system including insufficient respiratory drive (brain stem), impaired transmission of the breathing impulses (spinal cord, peripheral nerve), and morphological or functional abnormalities of the musculo-skeletal system of the thorax. Failure of the respiratory regulation on the one hand or execution of the impulses on the other hand represent different facets of the pathophysiology which can be described as “won’t breathe” and “can’t breathe” (2). Congenital central alveolar hypoventilation and opioid use represent examples of central disturbances, while amyotrophic lateral sclerosis (ALS), phrenic nerve palsy, muscle dystrophies, idiopathic sclerosis are examples of insufficient execution of ventilation. However, the obesity hypoventilation syndrome (OHS) combines components of both pathomechanisms. Chronic hypoventilation with daytime hypercapnia and sleep-related hypoventilation (SRH) do not differ substantially. Actually, SRH seems to represent an early stage of chronic hypoventilation. Nevertheless, this hypothesis has to be confirmed and the number of patients with advancing severity has to be evaluated in future research.
The most recent edition of the International Classification of Sleep Disorders (3) describes six subtypes of hypoventilation disorders in the chapter of sleep related breathing disorders:
- OHS;
- Congenital central alveolar hypoventilation syndrome (CCHS);
- Late-onset central hypoventilation with hypothalamic dysfunction;
- Idiopathic central alveolar hypoventilation;
- Sleep related hypoventilation due to a medication or substance;
- Sleep related hypoventilation due to a medical disorder.
The term CCHS replaces the name “Ondine’s curse”. CCHS requires evidence of a mutation of the PHOX2B-gene leading to a diffuse imbalance of the autonomic system. Differences in the mutation discriminate congenital from de novo development (4). Despite its congenital character, the disease may manifest in adulthood in some cases (3,5). Late-onset phenotypes may present with respiratory failure after general anesthesia, severe respiratory illness or respiratory depressants (6). However, in most cases hypoventilation begins during childhood and is more pronounced during sleep. The failure of central respiratory drive may be associated with respiratory rest during sleep. CCHS may be accompanied with other phenomena like Hirschsprung’s disease, cardiac arrhythmia, and tumors (6,7).
Idiopathic central alveolar hypoventilation is diagnosed if diseases of lung parenchyma, airways, pulmonary vessels, chest volume or neuromuscular diseases (NMD), drug treatment, obesity or congenital hypoventilation can be excluded (3,5). Several questions on idiopathic central alveolar hypoventilation are unresolved. The pathophysiology is unclear although an impairment of the hypercapnic and hypoxic chemoresponsiveness and respiratory drive has been discussed.
Late-onset central hypoventilation with hypothalamic dysfunction is a disorder of the central control of ventilation (3). It can be diagnosed if sleep related hypoventilation develops after the first years of life. The patients present with obesity, endocrine abnormalities of hypothalamic origin, severe emotional or behavioral disturbances or a tumor of neural origin (two of these four findings are required). Moreover, mutations of the PHOX2B-gene and other disorders explaining hypoventilation have to be excluded. Although the disease is associated with hyperphagia, hypoventilation persists even if patients lose weight. Diabetes insipidus, inappropriate antidiuretic hormone hypersecretion, precocious puberty, hypogonadism, hyperprolactinemia, hyperthyroidism and decreased growth hormone secretion are associated endocrine dysfunctions (3,5).
In contrast to these rare disorders, the OHS and chronic hypoventilation due to medical disorders or pharmaceutical influences represent the huge majority of chronic and SRH. OHS patients are characterized by obesity (BMI >30 kg/m2) and wakefulness hypercapnia. Hypoventilation in OHS cannot primarily be explained by other thoraco-pulmonary, neuromuscular or idiopathic diseases or pharmaceutical influences (3,5,8,9) (Table 1).

Full table
Sleep related hypoventilation due to a medical disorder is diagnosed in patients with underlying diseases of the lung parenchyma or the airways, the pulmonary vessels or neurological or musculo-skeletal disorders. In addition, SRH can be induced by drugs which depress ventilatory drive or impair muscle function. Long-acting narcotics, anesthetics, sedatives and muscle relaxants and also alcohol have been discussed (3,5). Chronic opioid intake may be associated with central apnoeas, atactic respiration but also sustained hypoxemia and hypoventilation (10).
Diagnosis of chronic and sleep related hypoventilation
Due to the broad variety of underlying diseases and pathophysiological mechanisms, there are no single typical clinical signs or symptoms which confidentially indicate or predict chronic or sleep related hypoventilation. Thus, a comprehensive clinical assessment, including a detailed history on sleep quality, morning symptoms, daytime fatigue or dyspnoea on exertion and a careful examination are crucial. Firstly, impaired alveolar ventilation becomes evident during sleep or exertion. Sleep is associated with a reduction of the minute ventilation even in healthy persons, while physical stress increases CO2 production. Hypoventilation during sleep may be associated with poor sleep quality, excessive daytime sleepiness and morning headaches. However, a relevant portion of patients reports no or only minor complaints. Typical clinical symptoms include reduced exercise capacity and dyspnoea. However, there are huge interindividual differences in clinical findings also depending on the underlying disease (6,11).
Chronic hypercapnic respiratory failure and hypoventilation during exertion can easily be diagnosed by arterial or capillary blood gas analysis during wakefulness. However, monitoring of respiration and carbon dioxide levels during sleep are needed to establish the diagnosis of SRH. Polysomnography (PSG) reveals the gold standard of investigating sleep and respiration. It is the only technique which allows to differentiate sleep and wakefulness and to diagnose electroencephalographic arousals and their relation to breathing disturbances. Sleep-wake transitions and arousals substantially influence respiration, leading to central breathing disturbances and propagation of periodic breathing (12-14). Thus, PSG is crucially important to precisely define the disease in individual patients and understand the underlying pathophysiology. Moreover, optimal therapy of chronic hypoventilation should not only focus on improvement of oxygen saturation and normalization of hypercapnia, but also in stabilization of the sleep profile. Therefore, we recommend PSG in the diagnostical work up of patients with chronic hypoventilation and in the follow-up of patients with persisting fatigue, sleepiness and morning headache under treatment. Nevertheless, if PSG is not available or cannot be performed due to comorbidities or complicated circumstances, multi-channel respiratory studies may suffice (15). When combined with actigraphy, they may also allow to separate sleep from wake periods (16).
Different invasive and non-invasive techniques are available to measure the carbon dioxide level. Arterial blood samples or samples from arterialized ear lobe represent the state of the art techniques for assessment of the PCO2. However, blood sampling during the night disrupts patients’ sleep, which may be associated with hyperventilation. Moreover, as a snapshot, single samples may not reflect the ventilator status of the whole night (17). Monitoring of the end-tidal CO2 (PetCO2) and of the transcutaneous CO2 (PtcCO2) allow for non-invasive and continuous measurement. PetCO2 is known to be influenced by nasal congestion and secretion. Moreover, it may substantially be limited by oxygen insufflation, non-invasive ventilation (NIV) and mask leaks (18,19). PtcCO2 allows for reliable and continuous measurement of the changes of the parameter. PtcCO2 correlates with PaCO2 although absolute figures often differ substantially. Actually, PtcCO2 represents a different parameter as it is influenced by the metabolism of the skin cells and the heating of the skin. Thus, PtcCO2 is systematically higher than PaCO2, which has to be considered when interpreting the results. In addition, there might be a shift of the PtcCO2 during long term measurements, although this problem seems to be less relevant with modern devices (17,20).
Numerous definitions of SRH have been introduced; they impair the comparison of studies and may have impact on clinical decisions. Previous definitions included increases of the PaCO2 >50 mmHg for >5% of measuring time or 10 mmHg rise or peak PtcCO2 >6.5 kPa (49 mmHg) (15). The most recent revision of the American Academy of Sleep Medicine (AASM) scoring criteria suggest to score SRH in case of:
- PCO2 >55 mmHg for ≥10 minutes during sleep or;
- Increases of the PCO2 ≥10 mmHg as compared to awake supine value up to a level of >50 mmHg for ≥10 minutes (21);
- In children, hypoventilation is scored if the PaCO2 or surrogate parameter increase >50 mmHg for >25% of total sleep time (21).
Hypoventilation is usually associated with a long term oxygen desaturation so that a decreased of the oxygen saturation (SaO2) <90% for >5 minutes with a nadir of ≤85% may also indicate hypercapnia and should urge to further diagnostical work up. Finally, as mentioned above, elevated levels of HCO3− after awakening suggest sleep hypoventilation even if the PaCO2 is within the normal range during wakefulness.
Identification of patients at risk for hypercapnic respiratory failure
An inversed breathing pattern in supine position may indicate a relevant deterioration of ventilatory muscle capacity in NMD. The change of the forced vital capacity (FVC) from erect to supine position may deliver additional information: while FVC decreases approximately by 8-10% from upright to supine position in healthy subjects, Allen et al. showed that a decrease >25% indicates an impaired diaphragmatic function (22). Ragette et al. found a correlation between inspiratory vital capacity (IVC) with respiratory muscle function and CO2 elimination in NMD during day and night. Onset of sleep disordered breathing was noticed with IVC <60%, while a figure below 40% was associated with continuous hypoventilation during sleep, respiratory failure both during sleep and wakefulness was likely below 25% (23). The sniff nasal pressure was superior to vital capacity (VC) in predicting reduced respiratory muscle strength in ALS without significant bulbar involvement (24). In addition, children with NMD exhibit significantly more often sleep related hypoventilation when they also suffer from scoliosis (25). Lung function parameters indicating a high respiratory load and low muscle capacity are major predictors of daytime hypercapnia in COPD (26). Forced expiratory volume in one second (FEV1) correlates with chronic hypercapnia in most studies. Montes de Oca and Celli studied 33 severe COPD patients, including 14 with daytime hypercapnia, and 20 controls. The likelihood of hypercapnia increased substantially if FEV1 was <0.5 liters (27). Hypercapnia was more probable in patients with a FEV1 <40% and in hyperinflation as demonstrated by Rodríguez-Roisin et al. (28). and Saure et al. (29).
Increased levels of HCO3− may indicate chronic or intermittent hypoventilation. It has been shown, that an elevated HCO3− level sufficiently predicts hypercapnia in obese subjects (30). The careful examination of the relation between PaCO2 and body-mass-index (BMI) may prevent underdiagnoses of OHS. Bülbül et al. found a ratio below 1.5 to be strongly predictive of the disease (31). In addition, oxygen saturation during sleep or wakefulness may also serve as an indicator of chronic hypercapnia. Basoglu et al. found an independent association of hypercapnia with reduced daytime SaO2 in OHS as compared to matched patients with obstructive sleep apnoea (OSA) (32). Parameters of lung function may reveal a restrictive pattern in morbidly obese patients including reduced VC and FEV1 which may be amplified in patients with OHS (33).
Pathophysiology of chronic hypoventilation
Although the reduction of the minute volume is the common characteristic of all chronic hypoventilation disorders, the underlying pathomechanisms differ substantially between the entities and can be complex in individual cases (exemplary shown for OHS in Figure 1).
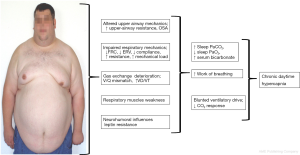
Respiratory mechanics importantly contribute to chronic hypoventilation in many conditions. Inspiratory muscle strength diminishes during the course of NMD with diaphragmatic involvement. Scoliosis and thoracic hyperkyphosis—idiopathic or secondary to NMD—may impair the diaphragm capacity and contribute to thoraco-pulmonary restriction.
Obesity adds additional mass load to the respiratory system and reduces lung volume, especially when severe and predominantly centrally distributed (34). It increases the resistance of the upper and lower airways and reduces the compliance of the respiratory system. Sharp et al. showed in the 1960s that the compliance is reduced by 60% in OHS patients as compared to non-obese patients and by 20% as compared to normocapnic obese patients (35). The small airways tend to collapse at low tidal volumes leading to trapping of the air and increasing intrinsic end-expiratory pressure (PEEPi) (36). All these factors elevate the work of breathing in OHS patients, both in upright and supine position (37). On the other hand, obesity reduces the expiratory reserve volume, leading to ventilation perfusion mismatch and abnormalities of the gas exchange (38).
Similar effects on PEEPi limit the VC in COPD patients with severe hyperinflation. The flattening of the diaphragm impairs its mechanical properties and increases the work of breathing (39).
In addition to alterations of the thoraco-pulmonary mechanics, the ventilatory control system contributes substantially to chronic hypercapnic failure and sleep related hypoventilation. While respiration is primarily driven by the carbon dioxide level in healthy subjects, the response to hypercapnia is usually altered in chronic hypercapnia. Radwan et al. compared patients with overlap of COPD and OSA with patients with OSA alone. Plasma bicarbonate concentration was significantly elevated in overlap patients, indicating chronic or long term nocturnal hypoventilation. Moreover, the hypercapnic ventilatory response was reduced in overlap patients, while it was normal in awake OSA patients (40).
Hypoxic and hypercapnic ventilatory response is also blunted in OHS patients as compared to normocapnic OSA patients (41). There is no evidence of impaired chemoresponsiveness in first degree relatives of OHS patients making an inherited condition improbable (42). However, the reduced chemoresponsiveness discriminates OHS patients from normocapnic obese individuals and OSA patients.
An impairment of the hypercapnic ventilatory response also contributes to the pathophysiology of NMD (15). This hypothesis is supported by findings of Nickol et al. in patients with hypercapnic respiratory failure due to restrictive thoracic diseases (NMD or chest wall disorders). Nocturnal NIV effectively controlled daytime PaCO2, although neither muscle strength, nor lung function or respiratory compliance significantly increased. Therefore, the authors concluded that the normalization of the chemosensitivity is the principal mechanism improving gas exchange under NIV (43).
The impairment of the central ventilatory drive may be the exclusive pathophysiological factor in rare cases, such as CCHS. They present with diminished minute ventilation both, during wakefulness and sleep which is most pronounced in non-rapid-eye-movement (NREM) sleep (44).
Leptin is a protein specifically produced by adipose tissue. Leptin crosses the blood-brain barrier and interacts with specific receptors in various areas of the brain and—among other effects—stimulates ventilation (45). Its contribution to the pathophysiology of OHS has intensively been discussed in recent years. The serum leptin concentration is elevated, associated with increased ventilation in obese individuals. This is thought to compensate for the increased CO2 production by excess body-mass (33). Shimura et al. compared circulating levels of leptin in OSA patients with and without hypercapnia. Serum leptin correlated with the BMI and was the only predictor for hypercapnia (46). However, leptin failed to adequately stimulate ventilation in hypercapnic individuals, which has been interpreted as a central leptin resistance contributing to the pathophysiology of OHS (11).
Obstructions of the upper airways additionally stress the ventilatory system and may impede CO2 elimination especially during sleep. Obstructive apnoeas and hypopnoeas are associated with transient episodes of acute hypercapnia. While eucapnic OSA patients hyperventilate between obstructive episodes and therefore eliminate the accumulated CO2 (47), the duration of the interval and increase of the minute ventilation may not allow for normalization of CO2 in patients with sleep related hypoventilation (48). Computer models suggest, that CO2 accumulates over the long term, when apnoea episodes become more than 3 times longer than the hyperventilation period between them (15). Obstructions of the upper airways may also be involved in patients with neuro-muscular diseases. The muscle of the upper airways may directly be involved if bulbar nerves and depending muscles are affected. The function of the upper airway muscles can be impaired in myopathic disorders or under pharmaceutical influences (long-term use of corticosteroids). Fat deposition and fluid retention may narrow the diameter of the upper airways. Peripheral edema and fluid overload frequently occur in patients with right heart failure in COPD or OHS. The fluid may shift from the lower limbs to the upper body components during recumbency. Redolfi et al. showed in non-obese men that the severity of OSA strongly correlates with the reduction of leg fluid volume and concomitant increase in neck circumference (49). In addition to the increased load to the uppper airways, they may collapse in central breathing disturbances (50). The capacity of the muscles may be insufficient to compensate for the additional load and the increased resistance in patients with NMD or thoraco-skeletal disorders.
As mentioned before, pharmaceutical therapy can also negatively impact ventilation. Steroid myopathy is an adverse effect of high dose, long-term use of systemic corticosteroids. Although corticosteroid-induced muscle atrophy affects predominantly type II b muscle fibers (51), additional effects on accessory respiratory muscles have to be discussed. Moreover, the long-term systemic treatment with glucocorticosteroids is a risk factor for the development and worsening of osteoporosis. The prevalence of osteoporosis in COPD varies between 9-69% and exceeds prevalence in healthy subjects (52). However, the causal relationship between corticosteroids and osteoporosis in COPD has not undoubtedly been demonstrated. Vertebral fractures due to osteoporotic sinterings and consecutive reductions of the weight diminish the efficiency of respiratory muscles. Recently, Watanabe et al. found an association between osteoporosis on the one hand and deterioration of pulmonary function on the other in Japanese male patients with COPD (53).
Opioids are often prescribed for chronic pain or palliation of dyspnoea in patients with severe, symptomatic lung disorders. They influence ventilation by blunting the hypercapnic ventilatory response, reducing breathing frequency, inducing the collapse of the upper airways and diminishing the activity of peripheral muscles (54). In addition to short term apnoeas and hypopnoeas, sustained hypoxia during sleep may delay arousals and increase the arousal threshold in NMD, COPD and OHS (55).
Influence of sleep on ventilation
Hypercapnia manifests during sleep prior to wakefulness as a consequence of physiological and pathophysiological changes of ventilation. The minute ventilation decreases from wakefulness to NREM sleep and further to rapid-eye-movement (REM) sleep by about 15% in healthy subjects (56).
The reduction of the minute ventilation during sleep is predominantly due to a lower tidal volume, which is not fully compensated by an increase of breathing frequency. The underlying pathophysiological mechanisms are complex: the tone of the thoracic muscles is reduced during sleep, reaching its lowest level during REM. The muscle relaxation also increases the upper airway resistance (50) and thus predisposes to upper airway obstruction. As discussed above, obstructive breathing disturbances account for further CO2 loading in obese patients and patients with NMD.
The muscle atonia during REM sleep affects primarily the accessory breathing muscles, whereas diaphragm contraction is saved. However, lung hyperinflation in COPD reduces the efficiency of the diaphragm, leading to a reduction of the tidal volume and the minute ventilation (9,57). It may also be reduced in diseases, in which accessory breathing muscles contribute substantially to ventilation.
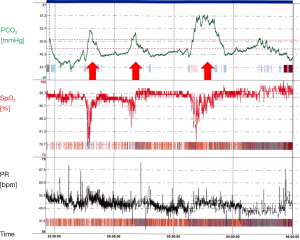
In addition, hypoxic and hypercapnic ventilatory responses are also blunted during REM sleep, leading to insufficient reactions to changes of the blood gases. Respiratory derailments during REM as well as NREM sleep trigger arousals, resulting in sleep fragmentation and diminished sleep efficacy. Thus, taking all these aspects together, sleep related hypoventilation manifests during REM sleep at first (Figure 2). PSG analyses in NMD showed a significant reduction of the REM proportion which might be regarded as protective mechanism to respiratory problems during REM sleep (58).
In clinical practice, it is not uncommon, that patients present with both, different entities of sleep related hypoventilation and other breathing disturbances during sleep. Ninety percent of OHS patients exhibit OSA (11). Nocturnal hypercapnia is more prevalent in COPD patients if they suffer from comorbid OSA (59). Their coexistence is referred to as the overlap syndrome and may be associated with pulmonary hypertension and right heart failure (60). Breathing disturbances in patients with Duchenne muscular dystrophy may begin with OSA (61), followed by sleep related hypoventilation when diaphragmatic weakness becomes critical and end with chronic hypoventilation. Several investigators have focused on breathing disturbances under chronic opioid use in recent years. Rose et al. showed chronic hypercapnic failure in patients with chronic pain and long term opioid therapy. In addition to hypoventilation, up to 50% of the patients presented with severe sleep apnoea, predominantly of central origin (62). CCHS is characterized by disturbed central chemical respiratory drive. However, central apnoeas and hypopnoeas have been described in PSG-studies of affected patients so that CCHS may present as predominant central sleep apnoea in individual cases (63).
Treatment of chronic hypoventilation
It is a general principle in medicine to treat any causative factors if possible. In terms of chronic hypoventilation this includes the cessation or reduction of drugs affecting breathing regulation, nerval transmission or muscular function. Opioids, benzodiazepine and other psychotropic drugs have to be reviewed critically. The muscles should be unloaded or their efficiency improved. Therefore, weight reduction, stabilization of vertebral fractures, orthopedic and surgical methods in kyphoscoliosis should be discussed. Electrical stimulation of the diaphragm can improve ventilation in individual cases (64,65).
However, causal treatment options will not suffice for the huge majority of patients with chronic or sleep related hypoventilation so that symptomatic therapy with mechanical ventilation becomes necessary. During the poliomyelitis pandemics in the 20th century, negative pressure ventilators—popularly named iron lungs, or steel cocoons—saved the life of thousands of patients afflicted with respiratory muscle paralysis (66). Negative pressure ventilation has completely been replaced by devices, which non-invasively apply ventilatory support via a nasal or oral-nasal mask to the patient. NIV generates the tidal volume by a fixed difference between inspiratory and expiratory pressure (pressure support, pressure controlled ventilation) or a predefined volume (volume support, volume controlled ventilation).
Optimal treatment of patients with chronic hypoventilation with NIV crucially depends on the underlying pathophysiological mechanisms. Algorithms primarily focusing on pressure support are most frequently used. They allow to separately adapting inspiratory and expiratory pressure and back-up frequency. The expiratory positive airway pressure (EPAP) is titrated according to the level of upper airway obstruction (12). It dilates the upper airways and therefore reduces the work of breathing to overcome upper airway resistance. In addition, the expiratory pressure stabilizes the small airways and may overcome the intrinsic peak, it increases air flow to atelectatic parts of the lungs and improves ventilation perfusion mismatch. The EPAP should be titrated under polygraphic or polysomnographic supervision, aiming at optimizing oxygen saturation, reducing apnoeas, hypopnoeas and flattening of the flow curve and respiration-related arousals. The improvement of sleep quality and the reduction of sleep-wake transitions avoid central breathing disturbances and stabilize ventilation (avoid periodic breathing) (12-14).
Tidal volume and breathing frequency influence the CO2 elimination and therefore counterbalance chronic or intermittent hypoventilation. Therefore, the inspiratory positive airway pressure (IPAP) is not the primary target of titration but the difference between EPAP and IPAP (Δ IPAP—EPAP) which defines the tidal volume. When EPAP has been titrated, the Δ IPAP—EPAP (the pressure support) can be adapted aiming at normalization of PaCO2 or PtcCO2.
The back-up frequency eliminates any central apnoeas and overcomes periods of bradypnoea. If the back-up frequency is set above the spontaneous breathing rate, the patient is fully controlled ventilated. In terms of patients’ compliance and synchronization, it is often reasonable to set the back-up frequency slightly below the spontaneous breathing frequency of the patient (67).
Taking these aspects together, the ventilator settings should be individually selected based on the underlying pathophysiological components (12):
- Airway obstruction can be addressed by increasing intraluminal expiratory pressure [CPAP, EPAP, bilevel in the spontaneous mode (BiPAP-S)];
- Reduced ventilatory drive (breathing frequency) can be counterbalanced by the application of mandatory breaths;
- Fixed or variable pressure support and mandatory breaths assure the necessary minute ventilation (12).
Controlled versus assisted NIV
Volume-controlled or pressure-controlled ventilation previously represented two extremes of NIV. While the former applied a fixed, predefined tidal volume, irrespective of the required inspiratory pressure level, the latter delivered a fixed pressure support, independent of the really applied volume. It has been hypothesized that optimal ventilation depends on maximal unloading of the respiratory muscles. This would allow restoring energy reserves required for spontaneous respiration. Controlled ventilation strategies unload respiratory muscles most intensively, as the ventilator takes over the complete work of breathing (68). However, synchronicity of the patients’ breathing rhythm with the ventilator may impact efficacy and tolerance. In addition, studies in animals and invasively ventilated intensive care patients have shown negative impact of controlled ventilation on muscle structure and function. In contrast, assisted ventilation allows the patient to spontaneously trigger pressure or volume support, which may improve synchronicity and may damage muscle fibers in less extent (69-71). In clinical practice, non-invasive pressure support ventilation has become the most favorite therapeutical approach. However, modern NIV devices work in hybrid modes: they apply a defined pressure support, a minimal preset tidal volume and minimal rate of mandatory breaths, combining the advantages and disadvantages of the algorithms.
Specific clinical situations may require variable ventilatory support, e.g., according to changing of the body position, sleep stage or patient’s respiratory drive. The most recent algorithms of NIV devices allow for automatically varying the expiratory pressure to overcome upper airway obstruction and adjusting the pressure support in order to ensure a predetermined target: pressure support ventilation with target volume (distributed as: average volume assured pressure support, AVAPS) (72-74). Nevertheless, the physician has to supervise the adaptation process and evaluate the effect on carbon dioxide, sleep parameters and upper airway obstruction. The automatic algorithms with a volume assurance may allow for individualized therapy but have not shown to be superior in general.
NIV in specific situations
NIV is indicated in neuromuscular and restrictive disorders and OHS if patients present with daytime hypercapnia >45 mmHg. In addition, ventilator support may be introduced in neuromuscular or thoraco-skeletal disorders in symptomatic nocturnal hypoventilation even without daytime hypercapnia (15). Keeping in mind that symptoms of nocturnal hypoventilation are highly variable and non-specific, a careful patient examination is necessary at each follow-up consultation. Additional factors emphasizing NIV include comorbidities with upper airway obstruction or impaired peak cough flow. NIV is indicated in COPD patients if they present with chronic daytime hypercapnia ≥50 mmHg or a PaCO2 of 46-50 mmHg associated with ≥2 hospitalizations within the last 12 months due to hypercapnic respiratory failure (75).
Volume-targeted algorithms may be favorable in NMD. These patients may suffer from impaired coughing causing mucoid bronchus obliteration so that pressure-targeted systems may not guarantee minimum minute ventilation (76). While patients with neuro-muscular or thoraco-skeletal diseases can often be treated sufficiently with low tidal volumes, high pressure support may be needed in patients with COPD. In both groups, the treatment target is normalization of carbon dioxide so that the term “high pressure ventilation” is misleading. Inspiratory pressure levels above 20 mbar are not primarily intended, but may be required to overcome hypercapnia. Dreher et al. compared this approach of high-intensity pressure (NPPV: mean inspiratory pressure 28.6±1.9 mbar) with low-intensity pressure (NPPV: mean inspiratory pressures of 14.6±0.8 mbar) in patients with severe stable hypercapnic COPD. The high-intensity regime was associated with better compliance (mean difference of 3.6 h/d) and was superior in terms of controlling nocturnal hypoventilation (77). Murphy et al. demonstrated that the pressure component is the most important factor in controlling hypoventilation, while changes in backup frequency (high versus low) did not relevantly impact PaCO2 (78).
Continuous positive airway pressure (CPAP) may be still the first therapeutical approach to patients with OHS. Due to the stabilization of upper airway obstruction, improvement of ventilation perfusion mismatch and lung mechanics, it might sufficiently normalize ventilation and oxygenation in a subgroup of OHS patients. Piper et al. randomized OHS patients to receive CPAP or bilevel ventilator support. They excluded patients with persisting severe nocturnal hypoxemia or sleep hypoventilation. Daytime carbon dioxide levels decreased similarly in both groups (79). However a substantial group of OHS patients did not sufficiently respond to CPAP, so that pressure support ventilation is the treatment of choice for the huge majority of patients with chronic hypoventilation. The heterogeneous responses to CPAP and pressure support ventilation might reflect the various contributions of the pathophysiological components (9). Similar to COPD, pressure support ventilation with volume assurance have not proven to generally be superior in OHS patients. Murphy et al. failed to demonstrate differences between automated volume assured pressure ventilation (AVAPS) and fixed-level pressure support in super obese patients (BMI 50±7 kg/m2) (72). However, hybrid modes and automatic algorithms may facilitate initiation and allow for individualized treatment (15).
NIV has proven to improve quality of sleep, nocturnal oxygen saturation, diurnal and nocturnal PaCO2 and quality of life in a broad spectrum of various chronic hypoventilation disorders (67). Moreover, NIV may improve patients’ survival (80). Non-invasive positive pressure ventilation added to standard treatment has proven to significantly improve survival in patients with COPD with PaCO2 ≥7 kPa (51.9 mmHg) (80). Bourke et al. demonstrated improvement of the survival by approximately 7 months in ALS with orthopnea or daytime hypercapnia (81). The medium age of death in Duchenne’s muscle dystrophy has ameliorated from 18-20 years to nearly 30 years under establishment of NIV (82). In addition, Nowbar et al. showed an increased mortality in patients with OHS. Twenty-three percent of OHS patients died within 18 months following hospital discharge as compared to 9% of patients with normocapnic obesity. Only 13% of the OHS patients were treated with NPPV. In addition, untreated OHS patients are more likely to require invasive ventilation and have prolonged hospital stays (83).
Most recently, a multicenter randomized controlled trial on the efficacy of NIV in severe stable COPD was performed. The investigators aimed at reducing hypercapnia by 20% or below a level of 48.1 mmHg. NIV was compared to standard treatment without ventilation. NIV significantly reduced 1 year mortality from 33% to 12% as compared to the control group. Therefore, from our point of view, NIV should be recommended to all COPD patients with chronic hypoventilation (PaCO2 ≥50 mmHg) (80,84).
Conclusions
The huge variety of underlying diseases and pathophysiological factors urge the clinician to individualize treatment. NIV has become the therapy of choice of chronic hypoventilation but has to be adapted according to the specific needs of the patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hudgel DW. Control of breathing. In: Weiss EB, Segal MS, Stein M, editors. Bronchial asthma: mechanisms and therapeutics. 2nd ed. Boston: Little, Brown and Co., 1984.
- Fahey PJ, Hyde RW. “Won’t breathe” vs “can’t breathe”. Detection of depressed ventilatory drive in patients with obstructive pulmonary disease. Chest 1983;84:19-25. [PubMed]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed (ICSD-3). Westchester: American Academy of Sleep Medicine, 2014.
- Amiel J, Laudier B, Attié-Bitach T, et al. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet 2003;33:459-61. [PubMed]
- Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest 2014;146:1387-94. [PubMed]
- Donic V, Tomori Z. Hypoventilation syndromes/chronic respiratory insufficiency in sleep. In: Simonds AK, De Backer JW, editors. ERS handbook respiratory sleep medicine. Sheffield: European Respiratory Society, 2012:48-51.
- Weese-Mayer DE, Silvestri JM, Huffman AD, et al. Case/control family study of autonomic nervous system dysfunction in idiopathic congenital central hypoventilation syndrome. Am J Med Genet 2001;100:237-45. [PubMed]
- Randerath WJ, Stieglitz S, Galetke W, et al. Pathophysiology of the obesity hypoventilation syndrome. Pneumologie 2008;62:398-403. [PubMed]
- Verbraecken J, McNicholas WT. Respiratory mechanics and ventilatory control in overlap syndrome and obesity hypoventilation. Respir Res 2013;14:132. [PubMed]
- Zutler M, Holty JE. Opioids, sleep, and sleep-disordered breathing. Curr Pharm Des 2011;17:1443-9. [PubMed]
- Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the-art review. Respir Care 2010;55:1347-62; discussion 1363-5. [PubMed]
- Randerath WJ. New ventilator support in complex phenotypes: coexisting CSA and OSA. ERS Monogr 2015;67:266-79.
- Eckert DJ, Jordan AS, Merchia P, et al. Central sleep apnea: Pathophysiology and treatment. Chest 2007;131:595-607. [PubMed]
- Ramirez JM, Garcia AJ 3rd, Anderson TM, et al. Central and peripheral factors contributing to obstructive sleep apneas. Respir Physiol Neurobiol 2013;189:344-53. [PubMed]
- Simonds AK. Chronic hypoventilation and its management. Eur Respir Rev 2013;22:325-32. [PubMed]
- Ramirez A, Khirani S, Delord V, et al. Assessment of sleep quality by pulse wave amplitude and actigraphy in children with sleep-disordered breathing: evaluation at diagnosis and under non-invasive ventilation. Sleep Breath 2013;17:827-35. [PubMed]
- Storre JH, Magnet FS, Dreher M, et al. Transcutaneous monitoring as a replacement for arterial PCO(2) monitoring during nocturnal non-invasive ventilation. Respir Med 2011;105:143-50. [PubMed]
- Schmitz BD, Shapiro BA. Capnography. Respir Care Clin N Am 1995;1:107-17. [PubMed]
- Schäfer T, Burmann-Urbanek M, Glaser S, et al. Multicenter-studie “Kapnographie in der Schlafmedizin”. Somnologie 1999;3:3-13.
- Randerath WJ, Stieglitz S, Galetke W, et al. Evaluation of a system for transcutaneous long-term capnometry. Respiration 2010;80:139-45. [PubMed]
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012;8:597-619. [PubMed]
- Allen SM, Hunt B, Green M. Fall in vital capacity with posture. Br J Dis Chest 1985;79:267-71. [PubMed]
- Ragette R, Mellies U, Schwake C, et al. Patterns and predictors of sleep disordered breathing in primary myopathies. Thorax 2002;57:724-8. [PubMed]
- Lyall RA, Donaldson N, Polkey MI, et al. Respiratory muscle strength and ventilatory failure in amyotrophic lateral sclerosis. Brain 2001;124:2000-13. [PubMed]
- Katz SL, Gaboury I, Keilty K, et al. Nocturnal hypoventilation: predictors and outcomes in childhood progressive neuromuscular disease. Arch Dis Child 2010;95:998-1003. [PubMed]
- Hillman D, Singh B, McArdle N, et al. Relationships between ventilatory impairment, sleep hypoventilation and type 2 respiratory failure. Respirology 2014;19:1106-16. [PubMed]
- Montes de Oca M, Celli BR. Mouth occlusion pressure, CO2 response and hypercapnia in severe chronic obstructive pulmonary disease. Eur Respir J 1998;12:666-71. [PubMed]
- Rodríguez-Roisin R, Drakulovic M, Rodríguez DA, et al. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol (1985) 2009;106:1902-8. [PubMed]
- Saure EW, Eagan TM, Jensen RL, et al. Explained variance for blood gases in a population with COPD. Clin Respir J 2012;6:72-80. [PubMed]
- Macavei VM, Spurling KJ, Loft J, et al. Diagnostic predictors of obesity-hypoventilation syndrome in patients suspected of having sleep disordered breathing. J Clin Sleep Med 2013;9:879-84. [PubMed]
- Bülbül Y, Ayik S, Ozlu T, et al. Frequency and predictors of obesity hypoventilation in hospitalized patients at a tertiary health care institution. Ann Thorac Med 2014;9:87-91. [PubMed]
- Basoglu OK, Tasbakan MS. Comparison of clinical characteristics in patients with obesity hypoventilation syndrome and obese obstructive sleep apnea syndrome: a case-control study. Clin Respir J 2014;8:167-74. [PubMed]
- Chau EH, Mokhlesi B, Chung F. Obesity Hypoventilation Syndrome and Anesthesia. Sleep Med Clin 2013;8:135-147. [PubMed]
- Steier J, Lunt A, Hart N, et al. Observational study of the effect of obesity on lung volumes. Thorax 2014;69:752-9. [PubMed]
- Sharp JT, Henry JP, Sweany SK, et al. The total work of breathing in normal and obese men. J Clin Invest 1964;43:728-39. [PubMed]
- Pankow W, Podszus T, Gutheil T, et al. Expiratory flow limitation and intrinsic positive end-expiratory pressure in obesity. J Appl Physiol (1985) 1998;85:1236-43. [PubMed]
- Lee MY, Lin CC, Shen SY, et al. Work of breathing in eucapnic and hypercapnic sleep apnea syndrome. Respiration 2009;77:146-53. [PubMed]
- Lin CK, Lin CC. Work of breathing and respiratory drive in obesity. Respirology 2012;17:402-11. [PubMed]
- Krieger BP. Hyperinflation and intrinsic positive end-expiratory pressure: less room to breathe. Respiration 2009;77:344-50. [PubMed]
- Radwan L, Maszczyk Z, Koziorowski A, et al. Control of breathing in obstructive sleep apnoea and in patients with the overlap syndrome. Eur Respir J 1995;8:542-5. [PubMed]
- Zwillich CW, Sutton FD, Pierson DJ, et al. Decreased hypoxic ventilatory drive in the obesity-hypoventilation syndrome. Am J Med 1975;59:343-8. [PubMed]
- Jokic R, Zintel T, Sridhar G, et al. Ventilatory responses to hypercapnia and hypoxia in relatives of patients with the obesity hypoventilation syndrome. Thorax 2000;55:940-5. [PubMed]
- Nickol AH, Hart N, Hopkinson NS, et al. Mechanisms of improvement of respiratory failure in patients with restrictive thoracic disease treated with non-invasive ventilation. Thorax 2005;60:754-60. [PubMed]
- Huang J, Colrain IM, Panitch HB, et al. Effect of sleep stage on breathing in children with central hypoventilation. J Appl Physiol (1985) 2008;105:44-53. [PubMed]
- Kalra SP. Central leptin insufficiency syndrome: an interactive etiology for obesity, metabolic and neural diseases and for designing new therapeutic interventions. Peptides 2008;29:127-38. [PubMed]
- Shimura R, Tatsumi K, Nakamura A, et al. Fat accumulation, leptin, and hypercapnia in obstructive sleep apnea-hypopnea syndrome. Chest 2005;127:543-9. [PubMed]
- Berger KI, Goldring RM, Rapoport DM. Obesity hypoventilation syndrome. Semin Respir Crit Care Med 2009;30:253-61. [PubMed]
- Ayappa I, Berger KI, Norman RG, et al. Hypercapnia and ventilatory periodicity in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2002;166:1112-5. [PubMed]
- Redolfi S, Yumino D, Ruttanaumpawan P, et al. Relationship between overnight rostral fluid shift and Obstructive Sleep Apnea in nonobese men. Am J Respir Crit Care Med 2009;179:241-6. [PubMed]
- Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev 2010;90:47-112. [PubMed]
- Gupta A, Gupta Y. Glucocorticoid-induced myopathy: Pathophysiology, diagnosis, and treatment. Indian J Endocrinol Metab 2013;17:913-6. [PubMed]
- Graat-Verboom L, Wouters EF, Smeenk FW, et al. Current status of research on osteoporosis in COPD: a systematic review. Eur Respir J 2009;34:209-18. [PubMed]
- Watanabe R, Tanaka T, Aita K, et al. Osteoporosis is highly prevalent in Japanese males with chronic obstructive pulmonary disease and is associated with deteriorated pulmonary function. J Bone Miner Metab 2015;33:392-400. [PubMed]
- Walker JM, Farney RJ, Rhondeau SM, et al. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. J Clin Sleep Med 2007;3:455-61. [PubMed]
- Hlavac MC, Catcheside PG, McDonald R, et al. Hypoxia impairs the arousal response to external resistive loading and airway occlusion during sleep. Sleep 2006;29:624-31. [PubMed]
- Douglas NJ, White DP, Pickett CK, et al. Respiration during sleep in normal man. Thorax 1982;37:840-4. [PubMed]
- Johnson MW, Remmers JE. Accessory muscle activity during sleep in chronic obstructive pulmonary disease. J Appl Physiol Respir Environ Exerc Physiol 1984;57:1011-7. [PubMed]
- Douglas NJ, White DP, Weil JV, et al. Hypercapnic ventilatory response in sleeping adults. Am Rev Respir Dis 1982;126:758-62. [PubMed]
- Chaouat A, Weitzenblum E, Krieger J, et al. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med 1995;151:82-6. [PubMed]
- Owens RL, Malhotra A. Sleep-disordered breathing and COPD: the overlap syndrome. Respir Care 2010;55:1333-44; discussion 1344-6. [PubMed]
- Khan Y, Heckmatt JZ. Obstructive apnoeas in Duchenne muscular dystrophy. Thorax 1994;49:157-61. [PubMed]
- Rose AR, Catcheside PG, McEvoy RD, et al. Sleep disordered breathing and chronic respiratory failure in patients with chronic pain on long term opioid therapy. J Clin Sleep Med 2014;10:847-52. [PubMed]
- Amimoto Y, Okada K, Nakano H, et al. A case of congenital central hypoventilation syndrome with a novel mutation of the PHOX2B gene presenting as central sleep apnea. J Clin Sleep Med 2014;10:327-9. [PubMed]
- Piper AJ, Grunstein RR. Big breathing: the complex interaction of obesity, hypoventilation, weight loss, and respiratory function. J Appl Physiol (1985) 2010;108:199-205. [PubMed]
- Onders RP. Functional electrical stimulation: restoration of respiratory function. Handb Clin Neurol 2012;109:275-82. [PubMed]
- Markel H. The genesis of the iron lung. Early attempts at administering artificial respiration to patients with poliomyelitis. Arch Pediatr Adolesc Med 1994;148:1174-80. [PubMed]
- Berry RB, Chediak A, Brown LK, et al. Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. J Clin Sleep Med 2010;6:491-509. [PubMed]
- Ward ME, Corbeil C, Gibbons W, et al. Optimization of respiratory muscle relaxation during mechanical ventilation. Anesthesiology 1988;69:29-35. [PubMed]
- Sassoon CS, Zhu E, Caiozzo VJ. Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med 2004;170:626-32. [PubMed]
- Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 2008;358:1327-35. [PubMed]
- Jubran A. Critical illness and mechanical ventilation: effects on the diaphragm. Respir Care 2006;51:1054-61; discussion 1062-4. [PubMed]
- Murphy PB, Davidson C, Hind MD, et al. Volume targeted versus pressure support non-invasive ventilation in patients with super obesity and chronic respiratory failure: a randomised controlled trial. Thorax 2012;67:727-34. [PubMed]
- Storre JH, Seuthe B, Fiechter R, et al. Average volume-assured pressure support in obesity hypoventilation: A randomized crossover trial. Chest 2006;130:815-21. [PubMed]
- Briones Claudett KH, Briones Claudett M, Chung Sang Wong M, et al. Noninvasive mechanical ventilation with average volume assured pressure support (AVAPS) in patients with chronic obstructive pulmonary disease and hypercapnic encephalopathy. BMC Pulm Med 2013;13:12. [PubMed]
- Windisch W, Walterspacher S, Siemon K, et al. Guidelines for non-invasive and invasive mechanical ventilation for treatment of chronic respiratory failure. Published by the German Society for Pneumology (DGP). Pneumologie 2010;64:640-52. [PubMed]
- Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation--a consensus conference report. Chest 1999;116:521-34. [PubMed]
- Dreher M, Storre JH, Schmoor C, et al. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 2010;65:303-8. [PubMed]
- Murphy PB, Brignall K, Moxham J, et al. High pressure versus high intensity noninvasive ventilation in stable hypercapnic chronic obstructive pulmonary disease: a randomized crossover trial. Int J Chron Obstruct Pulmon Dis 2012;7:811-8. [PubMed]
- Piper AJ, Wang D, Yee BJ, et al. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax 2008;63:395-401. [PubMed]
- Köhnlein T, Windisch W, Köhler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med 2014;2:698-705. [PubMed]
- Bourke SC, Tomlinson M, Williams TL, et al. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol 2006;5:140-7. [PubMed]
- Simonds AK, Muntoni F, Heather S, et al. Impact of nasal ventilation on survival in hypercapnic Duchenne muscular dystrophy. Thorax 1998;53:949-52. [PubMed]
- Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 2004;116:1-7. [PubMed]
- Elliott M. Domiciliary NIV for COPD: where are we now? Lancet Respir Med 2014;2:672-3. [PubMed]


