Vitamin D binding protein gene polymorphisms and chronic obstructive pulmonary disease: a meta-analysis
Introduction
Chronic obstructive pulmonary diseases (COPD) is a disease state characterized by not fully reversible and progressive airflow limitation, which is related to abnormal inflammatory response of the lungs to noxious particles or gases (1). Cigarette smoking is the most widely recognized risk factor for COPD, but at least one-quarter of COPD patients are non-smokers (2), and only 15% of smokers develop COPD, suggesting that genetic factors are involved in development of COPD (3). Besides, COPD is a familial clustering disease (4), it is reasonable to believe that COPD is the result of interactions of genetic factors and environmental factors. A registry-based twin study showed the familial aggregation of chronic bronchitis, particularly in women, which indicated the genetic origin of the increased susceptibility to respiratory disease among female (5). Another twin study also supported that the susceptibility to develop severe COPD is strongly influenced by genetic factors, and estimated that approximately 60% of the individual susceptibility can be explained by genetic factors from the data of 22,422 Danish twin pairs and 27,668 Swedish twin pairs with COPD (6). A Genome-wide association studies (GWAS), which evaluated 70,798 autosomal single nucleotide polymorphisms (SNPs) was conducted for measures of lung function in the Framingham Heart Study, suggested that SNP nearby vitamin D binding protein (VDBP) have an association with COPD (7). While in some other GWAS, the relationship between DBP and COPD was not found (8,9). Besides the Alpha 1-Antitrypsin gene, the only confirmed genetic factor (10), there are also some other genes associated with COPD, by involving in the pathogenesis of COPD (3,11-15). VDBP gene is one of the candidate genes associated with COPD (16), by implicating in macrophage activation and augmenting the chemotactic effect of complement-derived molecules on neutrophils (17), thus influencing the intensity of the inflammatory reaction.
VDBP, which is also known as Group-specific component (Gc-globulin, GC), has the function of binding substantial quantities of vitamin D and 25-hydroxyvitamin D (18). The human VDBP gene is localized on the long arm of chromosome 4 (4q12-q13), and it extends over 35 kb DNA and contains 13 exons and 12 introns. VDBP gene is highly polymorphic, with three common variants (GC1F, GC1S and GC2) and more than 124 rare variants (19). Two common point mutation (G→A, C→T) of SNPs (rs7041 and rs4588) in exon11 result in the three common isoforms and different protein products at positions 416 and 420: GC1F (Asp416, Thr 420), GC1S (Glu 416, Thr 420), and GC2 (Asp416, Lys420) (20,21).
To date, a series of case-control studies have been performed to investigate the relationship between VDBP gene polymorphisms and the risks of COPD, but the results were inconclusive. Some studies showed positive association between COPD risk and VDBP gene (4,21-28), but others offered negative results (12,13,29). Here, we undertook a meta-analysis to evaluate the association between DBP gene and COPD risk. To the best of our knowledge, there was no meta-analysis evaluating the relationship DBP gene polymorphisms and COPD risk before.
Materials and methods
Search strategy
We searched for studies evaluating the association between polymorphisms of the DBP gene and COPD risk. Articles were identified with a search of PubMed, EMBASE, Web of Science (Medline) and Chinese National Knowledge Infrastructure (CNKI) (last update February, 2015). The search terms used were as follows: [“chronic obstructive pulmonary disease” or “COPD” or “chronic bronchitis” or “emphysema” or “Pulmonary Disease, Chronic Obstructive (Mesh)”] and [“Group-specific component” or “Gc-globulin” or “vitamin D-binding protein” or “VDBP” or “‘Vitamin D-Binding Protein’ (Mesh)”]. There was no restriction on languages. References of the retrieved articles were also screened for additional studies.
Study selection
Studies were eligible for inclusion based on the following criteria: (I) studies assessed the association between DBP gene polymorphism and COPD susceptibility; (II) the design had to be a case-control study; (III) participants in control group were healthy individuals, who were excluded from COPD on the basis of history, symptom and spirometry; (IV) studies provided sufficient published data for estimating an odds ratio (OR) with a 95% confidence interval (95% CI). Articles were excluded based on the following condition: (I) family studies and affected compatriots studies, review articles, case reports and case series; (II) genotype frequencies in controls did not conform to Hardy-Weinberg equilibrium (HWE).
Data extraction
Two investigators extracted data from eligible studies independently and resolved controversies by discussion. For each report, we recorded first author, year of publication, ethnicity, diagnostic criteria of COPD, genotyping method, number and resources of cases and controls, mean age and smoking history (pack-years) of cases and controls, gender distribution, forced expiratory volume in one second (FEV1) and FEV1/forced vital capacity (FVC) ratio of cases and controls, allele and genotype frequency in cases and controls.
Quality score assessment
The Newcastle-ottawa quality assessment scale (NOS) was used to assess the quality of nonrandomized studies included (30). This scale judged the studies on three broad perspectives: selection, comparability and exposure. A “☆” rating system was used, and scores were ranged from 0 to 9. Studies with a score ≥7 were considered to be of high quality.
Statistical analysis and SROC analysis
VDBP gene has three distinct alleles in humans, namely GC1F, GC1S and GC2, which could be assembled into six different genotypes (1F-1F, 1F-1S, 1S-1S, 2-1S, 2-1F, 2-2). So the study was conducted using three different groups (GC1F/1S: 1F-1F, 1F-1S, 1S-1S; GC1F/2: 1F-1F, 1F-2, 2-2; and GC1S/2: 1S-1S, 1S-2, 2-2) following the traditional SNP polymorphism analysis. HWE in the control group was assessed using Chi-square test, with P≤0.05 considered statistically significant. This meta-analysis was conducted in dominant, recessive and co-dominant models, and one of them was selected to make the eventual meta-analysis (31). In the GC1F/1S group, OR1, OR2 and OR3 were calculated for genotypes 1F-1F vs. 1S-1S, 1F-1S vs. 1S-1S, and 1F-1F vs. 1F-1S, respectively. In the GC1F/2 group, OR1, OR2 and OR3 were calculated for genotypes 1F-1F vs. 2-2, 1F-2 vs. 2-2, and 1F-1F vs. 1F-2, respectively. In the GC2/1S group, OR1, OR2 and OR3 were calculated for genotypes 2-2 vs. 1S-1S, 1S-2 vs. 1S-1S, and 2-2 vs. 1S-2, respectively. The comparison between OR1, OR2 and OR3, and the P values were used to determine the most appropriate genetic models used in this meta-analysis. Besides, allele analysis (1F vs. 1S; 1F vs. 2; 1S vs. 2) and the comparison between the homozygotes and the other five genotypes (1F-1F vs. 1F-1S+1S-1S+2-1S+2-1F+2-2; 1S-1S vs. 1F-1F+1F-1S+2-1S+2-1F+2-2; 2-2 vs. 1F-1F+1F-1S+1S-1S+2-1S+2-1F) were conducted to assess the function of the three alleles and different homozygotes, respectively. The OR with 95% CI was used to assess the strength of the association between the DBP gene polymorphisms and COPD risk based on the genotype frequencies in cases and controls. Pooled ORs were calculated using fixed- or random-effect models. We used a Chi squared-based Q-test to assess heterogeneity among studies. P>0.05 was taken to suggest that effect sizes were larger than those expected by chance, and P≤0.05 indicated the strong heterogeneity. Therefore, when P>0.05, a pooled OR was calculated for each study using the fixed-effect model. Otherwise, the random effect model was used.
In order to assess the ethnicity-specific, subgroup analyses were performed by ethnicity. In order to assess the stability of the results, sensitivity analyses were performed through removing one study at a time.
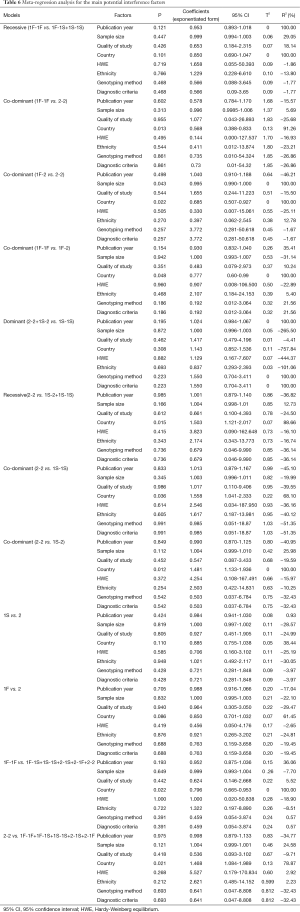
Meta-regression analyses were used to explore sources of heterogeneity across studies. Some factors, including publication year, sample size, quality of study, country, HWE, ethnicity, genotyping method [polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) or Isoelectric focusing electrophoresis] and diagnostic criteria of COPD (pulmonary function tests were included or not), were tested by meta-regression analysis in the most appropriate genetic model. The data were pooled using the DerSimonian and Laird random effects model (I2 >50%) or fixed effects model (I2 <50%) according to heterogeneity statistic I2. The values of P, Coefficients (exponentiated form) with 95% CI, Tau-squared (T2) and R2 were used in the test. The factor with P≤0.05 or R2 >5% was considered as the source of heterogeneity.
Funnel plots and Egger’s linear regression test were used to inspect the potential publication bias and P<0.05 was considered that significant publication bias existed.
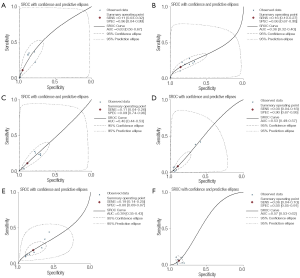
To evaluate the diagnosis potential of the polymorphism of VDBP to COPD, the summary receiver operating characteristic curve (SROC) analyses were conducted. The six different genotypes (1F-1F, 1F-1S, 1S-1S, 2-1S, 2-1F, 2-2) were considered as the different positive results of diagnostic test in each study. Values for sensitivity, specificity, true positive rate (TPR) and false positive rate (FPR) were produced from every study, and the plots were placed over the TPR and FPR points to form a smooth curve. A linear regression model was selected to fit the SROC curve where sensitivity and (1-specificity) are transformed into complex logarithmic variables. The exact area under the curve (AUC) for the SROC function was used to assess the accuracy of the test (32).
All statistical tests for this meta-analysis were performed using STATA 11.0 (StataCorp, College Station, USA).
Results
Characteristics of the studies
After a computerized search was performed, about 312 studies were identified. This list was reduced to 12 studies after screening the title and abstract. After we read the full texts of these articles, three of the 12 relevant articles were excluded because they did not present genotype frequency (13,26,33), and a total of nine articles were identified (21-25,27-29,34). The studies identified were published between 1990 and 2014, and the sample sizes ranged from 121 to 517. Of the nine studies, seven were published in English and other two in Chinese (22,28). These studies were performed in China (22,25,28), UK (21), Japan (23,24), Korea (34), Iceland (29) and Canada (27). The results from Chi-square tests showed that genotypic distribution of the controls was in agreement with the HWE except one study (24) (P=0.016) (Table 1). So we removed the data of both cases and controls in this study, and finally a total of eight case-control trials were included in the meta-analysis (Figure 1).

Full table
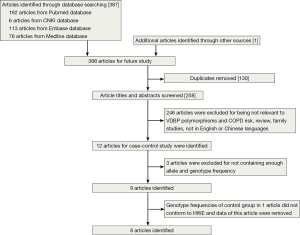
A total of 2,216 individuals (809 COPD patients and 1,407 control individuals) were included in this meta-analysis. Base on ethnicity, the participants were divided into two groups: 970 in Asian and 1,246 in Caucasians. All studies had a case-control design. All the studies used blood samples for DNA extraction. PCR-RFLP was the most commonly used genotyping method in these studies, except one study (27). The basic situations of the included studies and the allele and genotype frequency of the case groups and the control groups are shown in Table 1 and Table 2.

Full table
Of the eight studies included, most of the studies used the lung function tests as one of the criteria to define COPD, except one study (27), which appeared to use a diagnosis of chronic bronchitis or emphysema. In one study (34), most of the patients were in moderate to severe stage (186/203). Four studies included the moderate to severe COPD patients as the case groups (21,23,25,29). In one study (29), the phenotypes of the participants in case group were sub-classified into emphysema (33/102), bronchial hypersecretion with obstruction (BHO) (34/102), and chronic obstruction (CO) without hypersecretion (35/102), according to the radiological signs and sputum production. Other two studies offered no information about the severity of obstruction (22,28).
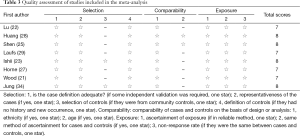
The NOS for assessing the quality of nonrandomized studies was shown in Table 3 and the scores ranged from 7 to 8. All of the studies were identified as relatively high-quality.
Full table
Selection of the most appreciated genetic model
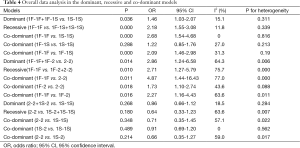
In the three groups (GC1F/1S, GC1F/2 and GC1S/2), overall data analysis were conducted in dominant, recessive and co-dominant models, respectively (Table 4). The genetic models were identified based on the comparison of ORs and the derived P values. In the GC1F/1S group, OR1 and OR3 were 2.68 and 2.09, respectively, with both P value <0.01. While OR2 was 1.22, with P value =0.29. Therefore, the recessive model (1F-1F vs. 1F-1S+1S-1S) was used in the following analysis. In the GC1F/2 group, OR1, OR2 and OR3 were 4.87, 1.73 and 2.27, respectively, with both P value <0.05. Therefore, the co-dominant model (1F-1F vs. 1F-2 vs. 2-2) was used in the following analysis. In the GC2/1S group, OR1, OR2 and OR3 were 0.71, 0.91 and 0.66, respectively, with both P value >0.05. Therefore, the three genetic models (dominant model: 2-2+1S-2 vs. 1S-1S; recessive model: 2-2 vs. 1S-2+1S-1S, and co-dominant model: 2-2 vs. 1S-2 vs. 1S-1S) were conducted.
Full table
Overall data analysis of the most appropriate genetic models
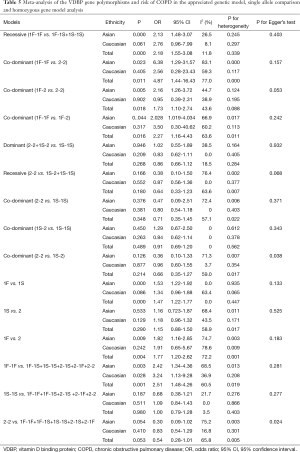
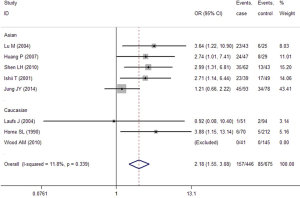
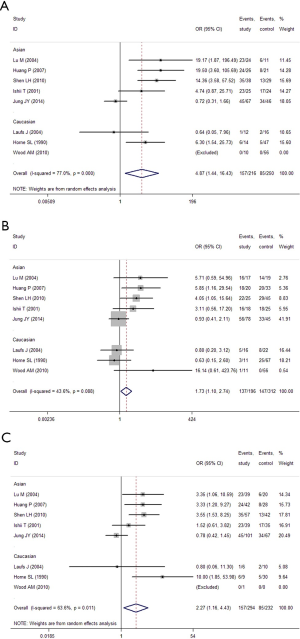
In the GC1F/1S group, recessive model was used, and the result (OR =2.18; 95% CI, 1.55-3.08; I2 =11.8%, fixed effects model) suggested that people who had genotypes 1F-1F had 1.18 times higher risk of COPD than people who had genotypes 1F-1S/1S-1S. Based on subgroup analysis, significant association were found in Asian (OR =2.13; 95% CI, 1.48-3.07; I2 =26.5%, fixed effects model). There was no evidence of a significant association in Caucasian (Table 5, Figure 2). In the GC1F/2 group, the co-dominant model was used, and the result of 1F-1F vs. 2-2 (OR =4.87; 95% CI, 1.44-16.43; I2 =77.0%, random effects model), 1F-2 vs. 2-2 (OR =1.73; 95% CI, 1.10-2.74; I2 =43.6%, fixed effects model) and 1F-1F vs. 1F-2 (OR =2.27; 95% CI, 1.16-4.43; I2 =63.6%, random effects model) suggested that people who had genotype 1F-1F and 1F-2 had 3.87 times and 0.73% higher risk of COPD than people who had genotypes 2-2, respectively, and that people who had genotypes 1F-1F had 1.27 times higher risk of COPD than people who had genotypes 1F-1S. Besides, subgroup analysis also showed significant association in Asian [(1F-1F vs. 2-2: OR =6.38; 95% CI, 1.29-31.57; I2 =83.1%, random effects model); (1F-2 vs. 2-2: OR =2.16; 95% CI, 1.26-3.72; I2 =44.7%, fixed effects model); (1F-1F vs. 1F-2: OR =2.028; 95% CI, 1.019-4.034; I2 =66.9%, random effects model)], but not in Caucasian (Figure 3, Table 5). In the GC2/1S group, all of the three genetic models were conducted, but no significant association was found. Besides, subgroup analysis could not find any significant association (Table 5).
Full table
A single allele (GC1F or GC1S or GC2) comparison
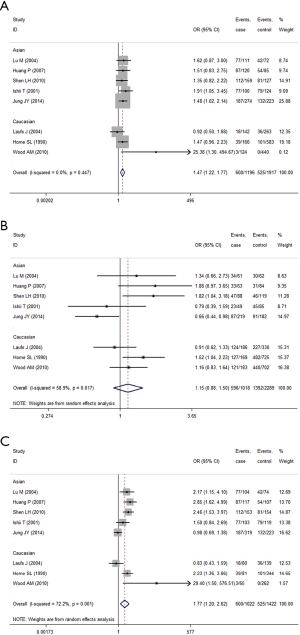
When the GC1F allele and GC1S allele, the summary OR was 1.47 (95% CI, 1.22-1.77; I2 =0.0%) in the fixed effects model, which also supported the hypothesis that the GC1F allele was a potential risk allele, and the GC1F allele had a 0.47 times higher risk of COPD than GC1S allele. For the GC1S allele was compared with GC2 allele, the result suggested no statistical difference between GC2 and GC1S allele in the protection of COPD (the random effects model: OR =1.15; 95% CI, 0.89-1.51; I2 =58.9%).When the GC1F allele was compared with GC2 allele, the result (the random effects model: OR =1.77; 95% CI, 1.20-2.62; I2 =72.2%) suggested that the GC1F allele had a 0.77 times higher risk of COPD than GC2 allele. Based on subgroup analysis, significant associations were found in Asian [(1F vs. 1S: OR =1.53; 95% CI, 1.22-1.92; I2 =0.0%); (1F vs. 2: OR =1.82; 95% CI, 1.16-2.85; I2 =74.7%)]. There was no evidence of a significant association in Caucasian (Figure 4, Table 5).
Analysis of homozygous genes (1F-1F or 1S-1S or 2-2) vs. other genotypes
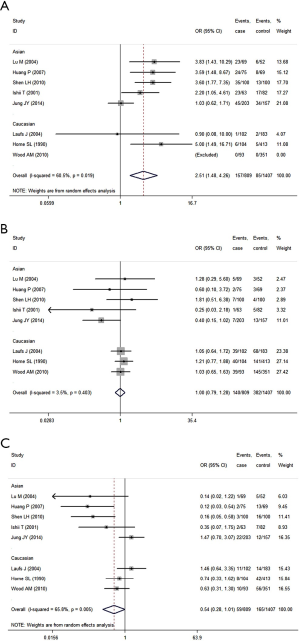
While analyzing samples using homozygous genotypes (1F-1F or 1S-1S or 2-2) in comparison with the five remaining genotypes in cases and controls, the OR was 2.51 (95% CI, 1.49-4.26; I2 =60.5%, random effects model) for GC1F-GC1F homozygotes, which suggested that people who had genotype 1F-1F had a 1.51 times higher risk of developing COPD than people who without this genotype. But no significant result was obtained in GC1S-GC1S and GC2-GC2 homozygotes. Based on subgroup analysis, significant associations were found in both Asian and Caucasian population for GC1F-GC1F homozygotes [(Asian: OR =2.42; 95% CI, 1.34-4.36; I2 =68.5%); (Caucasian: OR =3.24; 95% CI, 1.13-9.28; I2 =36.9%)] (Figure 5, Table 5).
When the genotypes 1F-1F, 1F-1S, 1F-2, 1S-1S, 1S-2 and 2-2 were considered as the positive result of diagnostic test, the AUCs of the SROCs were 0.63, 0.36, 0.48, 0.53, 0.39 and 0.57, respectively, showing less accurate performance of polymorphism of VDBP to COPD. The result also showed the better performance of GC1F-1F positive result to COPD than other genotypes (Figure 6).
Sensitivity analysis
Statistically similar results were obtained after sequentially excluding one study, suggesting the stability of the meta-analyses (Figure S1).

Meta-regression analysis
As shown in Table 6, in 1F-1F vs. 1F-1S+1S-1S model, publication year, country, sample size and quality of study could explain 100%, 100%, 29.05% and 18.14% of the heterogeneity, respectively. Besides, in 1F-1F vs. 2-2 model, sample size and country could explain 5.69% and 91.26% of the heterogeneity, respectively. While in 1F-2 vs. 2-2 model, sample size, country and ethnicity could explain 100%, 100% and 12.78% of the heterogeneity, respectively. The trend in OR was positively correlated with sample size (P=0.043). The result also indicated that in 1F-1F vs. 1F-2 model, quality of study, country, ethnicity, genotyping method and diagnostic criteria could explain 10.24%, 100%, 5.40%, 21.56% and 21.56% of the heterogeneity, respectively. Publication year, genotyping method and diagnostic criteria were main sources of the heterogeneity in 2-2+1S-2 vs. 1S-1S model (R2 =100%). In 2-2 vs. 1S-2+1S-1S model, sample size and country were indicated to explain 12.73% and 88.66% of the heterogeneity, respectively. In 2-2 vs. 1S-1S model, country was the source of heterogeneity (R2 =68.10%). Besides, in 2-2 vs. 1S-2 model, sample size (R2=25.98%) and country (R2=100%) were the sources of heterogeneity. The result also indicated that country was the source of heterogeneity (R2 =38.44%) in 1S vs. 2 model. Besides, in 1F vs. 2 model, country was indicated as the source of heterogeneity (R2 =61.45%). In 1F-1F vs. 1F-1S+1S-1S+2-1S+2-1F+2-2 model, publication year, quality of study and country could explain 36.06%, 5.52% and 100.00% of the heterogeneity, respectively. In 2-2 vs. 1F-1F+1F-1S+1S-1S+2-1S+2-1F model, sample size (R2 =24.58%) and country (R2 =78.87%) were indicated as the source of heterogeneity (Table 6). The meta-regression analysis in 1S-2 vs. 1S-1S model, 1F vs. 1S model and 1S-1S vs. 1F-1F+1F-1S+2-1S+2-1F+2-2 model were not conducted because of the absence of statistical heterogeneity (Figure 6).
Full table
Publication bias
The shape of the funnel plots was symmetrical for most of the results, and the statistic results of Egger’s linear regression test also indicated a lack of publication bias (P>0.05) expect in 2-2 vs. 1S-2 (P=0.038) and 2-2 vs. 1F-1F+1F-1S+1S-1S+2-1S+2-1F (P=0.024) (Table 5).
Discussion
This study provided the first meta-analysis result of a contribution of the VDBP gene to COPD susceptibility. In this meta-analysis, the results of single allele comparison suggested that GC1F is a risk factor of COPD, GC2 and GC1S allele are protective factors for COPD. In the overall data analysis of the most appropriate genetic models, we found that the carriers of the GC1F allele had a higher risk, and the carriers of the GC2 and GC1S allele played a protect role in COPD. The results of homozygous gene research groups also supported that the GC1F-GC1F polymorphism contributed to COPD as a risk factor.
In the present study, overall data analyses were conducted in dominant, recessive and co-dominant models, and one of them was selected to make the eventual meta-analysis. The determination of the most appropriate genetic model is partly dependent on the numbers in each genotype group, which in turn is dependent on allele frequencies. Considering that low numbers in a some genotype groups may lower the power to detect a particular model, the statistical power to indicate a particular mode of inheritance should be checked (31). Therefore, it is very important to select the most appropriate genetic model for meta-analysis.
Heterogeneity is a potential problem when interpreting the results of a meta-analysis. The heterogeneity arises when the effects in the respective study populations are not the same, and it can influence meta-analysis result (35). Therefore, it was important to find the sources of heterogeneity. In the present meta-analysis, significant heterogeneities were found in most of the comparison models (P values of heterogeneity test for 1F-1F vs. 2-2, 1F-1F vs. 1F-2, 2-2 vs. 1S-2+1S-1S, 2-2 vs. 1S-1S, 2-2 vs. 1S-2, 1S vs. 2, 1F vs. 2, 1F-1F vs. 1F-1S+1S-1S+2-1S+2-1F+2-2 and 2-2 vs. 1F-1F+1F-1S+1S-1S+2-1S+2-1F were all less than 0.05, and I2 values were larger than 50%). To find the sources of heterogeneity, Meta-regression analysis was performed. In the comparison models with heterogeneities, the result showed publication year, sample size, quality of study, country, ethnicity, genotyping method and diagnostic criteria of COPD were the most important heterogeneity sources, while HWE could not explain the heterogeneity (Table 6).
In this study, we also conducted the SROC analyses to evaluate the diagnosis potential of the polymorphism of VDBP to COPD, and the AUCs were used to assess the accuracy of the test. The diagnosis abilities of the six different genotypes (1F-1F, 1F-1S, 1S-1S, 2-1S, 2-1F, 2-2) were low. However, we could find that GC1F-1F offered the best diagnosis performance to COPD, which supported the result of our meta-analysis that GC1F allele could be a risk factor for COPD.
Many reports have suggested the potential mechanism of the association between GC gene polymorphisms and COPD. Vitamin D deficiency is common in COPD patients and correlates with severity of COPD (21,33). While in contrast to vitamin D, circulating DBP deficiency is inversely related to low FEV1 (21), therefore, DBP may play an important role in mechanism of COPD. DBP is a 55 kDa protein, which expressed not only in liver, kidney, gonads and fat, but also by neutrophils (20). Firstly, it was confirmed that DBP plays a pivotal role in modulating monocyte responses to 25OHD3, and that it is associated with its deglycosylation (36). Therefore, these effects vary according to DBP genotype. The GC2 variant is less able to activate macrophages, for the absence of a glycosylated residue at position 420 (21), so it plays a protectional role in inflammation. However, GC1F plays an opposite effect because of its different protein product at position 420 (16). So the different effect may due to the difference of Gc protein oligosaccharide structure (37). Secondly, DBP can enhance neutrophil chemotactic activity of complement derived C5a and C5a des Arg, which is associated with inflammation (38). But no significant different neutrophil chemotaxis is found in different allele (GC1F, GC1S and GC2).
Subgroup analysis by ethnicity allowed us to look for potential ethnic differences. In Asian population, the result of the five articles (including 510 cases and 460 controls) suggested that the GC1F allele was associated with increased risk of COPD and that GC2 and GC1S allele were protective factors of COPD based on allelic contrast and dominant contrast, recessive contrast and homozygote comparison. However, for the Caucasian population, three articles (299 cases and 947 controls) were included, and significant association was only found in 1F-1F vs. 1F-2+2-2, suggesting that 1F homozygote may be a risk factor for COPD in the Caucasian when compared with GC2. We found that the association between GC polymorphisms and COPD risk varied between ethnicities. The reasons causing the difference may be as followed. Firstly, subgroup analyses identified only a small number of studies, which may have resulted in poor statistical power. Secondly, one of the three Caucasian studies used chronic bronchitis and emphysema as diagnostic criteria without the spirometric criteria, the lack of significant findings in the Caucasian may be attributed to this diagnostic criteria. Thirdly, the sensitivity of individuals to COPD may be affected by different genetic backgrounds and degrees of environmental exposure.
Some limitations of this meta-analysis should be acknowledged. Firstly, all available literature should be included in the meta-analysis, but articles were only identified with a search of PubMed and CNKI, and we only included literature published in English and Chinese, thus neglecting studies published in other languages. Besides, all the studies included were from the Asian and Caucasian, and most of the significant findings occurred only in the Asian, thus limiting the generalizability of the result. Further studies are required in other ethnic groups, such as Africans and Latinos. Secondly, only three studies are in the Caucasian, and one of them uses a diagnosis of chronic bronchitis or emphysema, without the spirometric criteria, which may lead the result to be underpowered. Thirdly, significant heterogeneities were still found in a couple of comparisons. After stratified analyses by ethnicity, heterogeneities were partly reduced or removed, suggesting that ethnicity could explain part of the heterogeneities. Besides, the meta-regression analysis could find the sources of heterogeneity. Lastly, data were only stratified by ethnicity without other factors, such as age and gender, smoking history, and phenotype of the disease, as sufficient information could not be extracted from the studies.
Although there are some limitations, this is the first meta-analysis concerning the relationship between GC gene polymorphisms and COPD risk to date. Our results reveal that the GC1F allele may be a risk factor for COPD, while the presence of the GC2 and GC1S allele may be protective factors against COPD, especially in Asian population. Our results reveal no association between GC gene polymorphisms and COPD in the Caucasian, so further case-control studies are needed.
Acknowledgements
Funding: This work was supported by Science and Technology Program of Health Department, Guangxi Zhuang Autonomous Region (NO. Z2011314).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256-76. [PubMed]
- Zeng G, Sun B, Zhong N. Non-smoking-related chronic obstructive pulmonary disease: a neglected entity? Respirology 2012;17:908-12. [PubMed]
- Bossé Y. Genetics of chronic obstructive pulmonary disease: a succinct review, future avenues and prospective clinical applications. Pharmacogenomics 2009;10:655-67. [PubMed]
- Kueppers F, Miller RD, Gordon H, et al. Familial prevalence of chronic obstructive pulmonary disease in a matched pair study. Am J Med 1977;63:336-42. [PubMed]
- Meteran H, Backer V, Kyvik KO, et al. Heredity of chronic bronchitis: a registry-based twin study. Respir Med 2014;108:1321-6. [PubMed]
- Ingebrigtsen T, Thomsen SF, Vestbo J, et al. Genetic influences on Chronic Obstructive Pulmonary Disease - a twin study. Respir Med 2010;104:1890-5. [PubMed]
- Wilk JB, Walter RE, Laramie JM, et al. Framingham Heart Study genome-wide association: results for pulmonary function measures. BMC Med Genet 2007;8:S8. [PubMed]
- Repapi E, Sayers I, Wain LV, et al. Genome-wide association study identifies five loci associated with lung function. Nat Genet 2010;42:36-44. [PubMed]
- Loth DW, Artigas MS, Gharib SA, et al. Genome-wide association analysis identifies six new loci associated with forced vital capacity. Nat Genet 2014;46:669-77. [PubMed]
- Sandford AJ, Weir TD, Paré PD. Genetic risk factors for chronic obstructive pulmonary disease. Eur Respir J 1997;10:1380-91. [PubMed]
- Sampsonas F, Karkoulias K, Kaparianos A, et al. Genetics of chronic obstructive pulmonary disease, beyond a1-antitrypsin deficiency. Curr Med Chem 2006;13:2857-73. [PubMed]
- Sandford AJ, Paré PD. Genetic risk factors for chronic obstructive pulmonary disease. Clin Chest Med 2000;21:633-43. [PubMed]
- Hersh CP, Demeo DL, Lange C, et al. Attempted replication of reported chronic obstructive pulmonary disease candidate gene associations. Am J Respir Cell Mol Biol 2005;33:71-8. [PubMed]
- Marciniak SJ, Lomas DA. What can naturally occurring mutations tell us about the pathogenesis of COPD? Thorax 2009;64:359-64. [PubMed]
- Joos L, Paré PD, Sandford AJ. Genetic risk factors of chronic obstructive pulmonary disease. Swiss Med Wkly 2002;132:27-37. [PubMed]
- Bakke PS, Zhu G, Gulsvik A, et al. Candidate genes for COPD in two large data sets. Eur Respir J 2011;37:255-63. [PubMed]
- Speeckaert M, Huang G, Delanghe JR, et al. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta 2006;372:33-42. [PubMed]
- Daiger SP, Schanfield MS, Cavalli-Sforza LL. Group-specific component (Gc) proteins bind vitamin D and 25-hydroxyvitamin D. Proc Natl Acad Sci USA. 1975;72:2076-80. [PubMed]
- Cleve H, Constans J. The mutants of the vitamin-D-binding protein: more than 120 variants of the GC/DBP system. Vox Sang 1988;54:215-25. [PubMed]
- Chishimba L, Thickett DR, Stockley RA, et al. The vitamin D axis in the lung: a key role for vitamin D-binding protein. Thorax 2010;65:456-62. [PubMed]
- Wood AM, Bassford C, Webster D, et al. Vitamin D-binding protein contributes to COPD by activation of alveolar macrophages. Thorax 2011;66:205-10. [PubMed]
- Lu M, Yang B, Cai YY. The relationship between vitamin D binding protein gene polymorphism and chronic obstructive pulmonary disease. Zhonghua Nei Ke Za Zhi 2004;43:117-20. (in Chinese). [PubMed]
- Ishii T, Keicho N, Teramoto S, et al. Association of Gc-globulin variation with susceptibility to COPD and diffuse panbronchiolitis. Eur Respir J 2001;18:753-7. [PubMed]
- Ito I, Nagai S, Hoshino Y, et al. Risk and severity of COPD is associated with the group-specific component of serum globulin 1F allele. Chest 2004;125:63-70. [PubMed]
- Shen LH, Zhang XM, Su DJ, et al. Association of vitamin D binding protein variants with susceptibility to chronic obstructive pulmonary disease. J Int Med Res 2010;38:1093-8. [PubMed]
- Schellenberg D, Paré PD, Weir TD, et al. Vitamin D binding protein variants and the risk of COPD. Am J Respir Crit Care Med 1998;157:957-61. [PubMed]
- Horne SL, Cockcroft DW, Dosman JA. Possible protective effect against chronic obstructive airways disease by the GC2 allele. Hum Hered 1990;40:173-6. [PubMed]
- Huang P, Ma Y, Du X, et al. Vitamin D binding protein gene polymorphisms in COPD patients. Chin J Tuberc Respir Dis 2007:780-1.
- Laufs J, Andrason H, Sigvaldason A, et al. Association of vitamin D binding protein variants with chronic mucus hypersecretion in Iceland. Am J Pharmacogenomics 2004;4:63-8. [PubMed]
- Zhang R, Wang J, Yang R, et al. Effects of Pro12Ala polymorphism in peroxisome proliferator-activated receptor-γ2 gene on metabolic syndrome risk: a meta-analysis. Gene 2014;535:79-87. [PubMed]
- Thakkinstian A, McElduff P, D'Este C, et al. A method for meta-analysis of molecular association studies. Stat Med 2005;24:1291-306. [PubMed]
- Midgette AS, Stukel TA, Littenberg B. A meta-analytic method for summarizing diagnostic test performances: receiver-operating-characteristic-summary point estimates. Med Decis Making 1993;13:253-7. [PubMed]
- Janssens W, Bouillon R, Claes B, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 2010;65:215-20. [PubMed]
- Jung JY, Choi DP, Won S, et al. Relationship of vitamin D binding protein polymorphisms and lung function in Korean chronic obstructive pulmonary disease. Yonsei Med J 2014;55:1318-25. [PubMed]
- Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ 2007;335:914-6. [PubMed]
- Chun RF, Lauridsen AL, Suon L, et al. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab 2010;95:3368-76. [PubMed]
- Ohkura K, Nagasawa H, Uto Y, et al. The role of Gc protein oligosaccharide structure as a risk factor for COPD. Anticancer Res 2006;26:4073-8. [PubMed]
- Kew RR, Webster RO. Gc-globulin (vitamin D-binding protein) enhances the neutrophil chemotactic activity of C5a and C5a des Arg. J Clin Invest 1988;82:364-9. [PubMed]


