Thymic carcinoma developing in a multilocular thymic cyst
Abstract
Thymic carcinoma developing in a pre-existing thymic cyst has rarely been reported in literature. The diagnosis of this entity in the past has always been established after surgery. We are reporting a case of thymic carcinoma that developed in a pre-existing multilocular thymic cyst in an elderly male. The diagnosis was based on imaging findings and confirmed on fine needle aspiration cytology (FNAC).
Key words: Thymic cyst; carcinoma; fine needle aspiration cytology
Introduction
Cysts of thymus are rare and can be congenital or acquired (1-3). Congenital cysts are unilocular, whereas, the acquired cysts are usually multilocular and results as a consequence of inflammatory processes (1-4). The lesions are usually suspected on chest radiograph and confirmed on cross sectional imaging. Occasionally, curvilinear calcifications within the anterior mediastinal mass may be seen on radiographs suggesting possibility of cystic calcifications (5). Thymic carcinoma developing in a pre-existing thymic cyst is a rare entity, and only few case reports are present in the English literature (6-10). Presence of soft tissue component within the thymic cysts does not warrant the diagnosis of malignancy as these may normally be present within the cysts due to previous inflammation (4). Hence, it is not surprising that the reported cases of malignant transformation of thymic cysts were diagnosed only after histopathological examination of resected specimens.
Case report
A 63 years old male, chronic smoker for last 25 years presented with dyspnoea associated with cough and expectoration for last 5-6 years. There was no history of fever, loss of weight or appetite, hemoptysis, diabetes mellitus, hypertension or pulmonary tuberculosis. On examination he was conscious, oriented with pulse of 70/min, blood pressure 130/80 mmHg. Respiratory examination revealed dullness in left infraclavicular region with decreased air entry on left side.
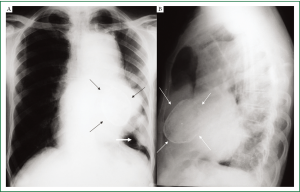
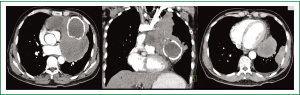
Complete blood counts, renal and liver function tests were within normal limits. Chest radiographs showed a large anterior mediastinal mass silhouetting left cardiac border with calcified cyst in its inferior aspect. In addition, a peripheral pleural based soft tissue mass was seen in the left lower zone (Figure 1A,B). Contrast enhanced CT (CECT) of chest showed a large multicystic lesion in the anterior mediastinum with one of the cyst showing curvilinear calcification in its wall. A large intracystic soft tissue component was seen in one of the cyst which was extending outside obscuring the intervening fat planes with the pulmonary arteries, left main bronchus and pericardium (Figure 2A,B). A pleural based soft tissue mass was also seen in the left hemi-thorax (Figure 2C). There were no mediastinal or hilar lymphadenopathy, focal lung lesion or pleural effusion. The radiological picture was consistent with a malignant anterior mediastinal mass with pleural based metastatic deposit. In view of presence of curvilinear calcification within the mass a possibility of multinodular goiter with retrosternal extension, a malignant teratoma or multilocular thymic cyst was considered. However, there was no direct continuation of the mass with the thyroid and presence of mediastinal invasion and pleural based deposit pointed towards a malignant etiology. Serum levels of human chorionic gonadotropin (HCG), alpha-fetoprotein (AFP), anti-acetylcholine receptor antibody, carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9), carbohydrate antigen 125 (CA 125) were all within the normal range. HIV serology was non reactive.
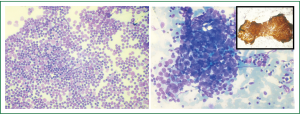
A percutaneous ultrasound guided FNAC from soft tissue component of the mediastinal mass through the left para-sternal intercostal space was performed and the pleural deposit was separately sampled. Air-dried smears and alcohol-fixed smears were stained with May-Grϋnwald-Giemsa stain and hematoxyline-Eosin stain respectively. The smears from mediastinal mass aspirate showed numerous clusters of epithelial cells displaying mild to moderate atypia in a background of abundant reactive lymphoid tissue (Figure 3A). Hence a cyto-pathological diagnosis consistent with thymoma B3 as per WHO classification was made. The aspirate from the pleural deposit also showed cells with similar morphology confirming metastasis (Figure 3B). Immunoperoxidase staining was performed with mouse anti-pancytokeratin antibody (Dakopatts, Sweden) on the alcohol-fixed smear. The thymic epithelial cells in the aspirate showed strong cytokeratin immunoreactivity (inset Figure 3B) confirming metastases from the thymic carcinoma. In view of the metastatic deposit and the radiological findings, the diagnosis was consistent with thymic carcinoma developing in multilocular thymic cyst (Masaoka Stage IVa). Since the tumor was unresectable chemotherapy- PAC regimen (cisplatin, doxorubicin, cyclophosphamide) was started to which patient showed partial response and expired after 14 months.
Discussion
Thymic cysts are either congenital or acquired in origin (1-3). The congenital cysts are typically unilocular, contain clear fluid, have walls that are thin to the point of translucency, and show no evidence of inflammation on histopathogic examination (1,2). In contrast, acquired thymic cysts results from an inflammatory process and are usually multi-locular (2-4). The cysts contain turbid fluid or gelatinous material, have walls that are thick and fibrous, and typically have evidence of significant inflammation and fibrosis on histopathogic examination (2). Soft tissue attenuation intracystic component may be seen which does not necessarily indicate malignancy and tissue sampling may be necessary to distinguish them from cystic thymoma. Cystic thymoma in contrast to non-neoplastic cysts demonstrate reticuloepithelial and/or mature lymphocytes on FNAC with absence of epithelial lining and perivascular spaces and foci of medullary differentiation on resected specimens (11). Multi-locular thymic cysts should be distinguished from congenital cysts for several reasons: they may recur after excision, they may be associated with thymic neoplasms such as thymoma or thymic carcinoma, and they may adhere to adjacent structures and simulate an invasive neoplasm at thoracotomy (2). Most cases of acquired thymic cysts are seen in the setting of HIV infection (12). The etiology of multilocular thymic cyst in our case could not be ascertained as the patient did not have any autoimmune disease and was tested negative for HIV.
Thymic carcinoma developing in the setting of acquired thymic cyst is a rare occurrence (6-13). In most cases, both thymic carcinoma and thymic cyst are asymptomatic and diagnosed only when these compress juxtaposed mediastinal structures like pulmonary vessels or bronchi (7,13). Thymic carcinoma per se is rarely associated with myasthenia gravis or other immune mediated systemic diseases.
Presence of multiple cysts within anterior mediastinal mass can be seen in multinodular goiter with retrosternal extension, germ cell tumours, lymphomas, and thymic multilocular cyst. As lymphomas without treatment do not show calcification and the pattern of curvilinear calcification is virtually never seen even after treatment of lymphoma, this possibility was not considered in our case. A retrosternal extension of multinodular goiter was also ruled out as the mass was discontinuous with normally placed thyroid in neck. In adults, germ cell tumors occur most commonly between the second and fourth decades of life and are found in equal numbers in both sexes, although malignant germ cell tumors are almost exclusively seen in males. Malignant germ cell tumors are subdivided into seminomas and nonseminomatous. Nonseminomatous tumors also are termed malignant teratomas and are divided further by cell type into choriocarcinomas, embryonal carcinomas, mixed tumors, teratocarcinomas, and yolk sac carcinomas. Approximately 95% of patients with germ cell tumors of the mediastinum have an elevated tumor marker like, alpha-fetoprotein (αAFP), human chorionic gonadotropin (βhCG) and Serum lactic dehydrogenase (LDH). In view of possible diagnosis of malignant germ cell tumor, the various tumor markers viz. βHCG, αAFP, and LDH were assessed in our case, but were within normal limits. FNAC of the mediastinal mass in our case were conclusive of thymoma. The cytology has its limitation in differentiating thymic carcinoma from thymoma. A diagnosis of thymic carcinoma is possible when the radiological findings are suggestive of mediastinal invasion or metastasis. The admixed of malignant epithelial cells along with reactive lymphoid cells was compatible with type B3 thymoma as per WHO classification. Further, metastasis was confirmed from FNAC of pleural based mass confirming the diagnosis of thymic carcinoma.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Indeglia RA, Shea MA, Grage TB. Congenital cysts of the thymus gland. Arch Surg 1967;94:149-52.
- Suster S, Rosai J. Multilocular thymic cyst: an acquired reactive process—study of 18 cases. Am J Surg Pathol 1991;15:388-98.
- Jeung MY, Gasser B, Gangi A, Bogorin A, Charneau D, Wihlm JM, et al. Imaging of cystic masses of the mediastinum. Radiographics 2002;22:S79-93.
- Choi YW, McAdams HP, Jeon SC, Hong EK, Kim YH, Im JG, et al. Idiopathic multilocular thymic cyst: CT features with clinical and histopathological correlation. AJR Am J Roentgenol 2001;177:881-5.
- Strollo DC, Rosado de Christenson ML, Jett JR. Primary mediastinal tumors. Part 1: tumors of the anterior mediastinum. Chest 1997;112:511-22.
- Yamashita S, Yamazaki H, Kato T, Yokota T, Matsumoto N, Matsukura S. Thymic carcinoma which developed in a thymic cyst. Internal Medicine 1996;35:215-8.
- Ucvet A, Yuncu G, Sevinc S, Ceylan KC, Ozsinan F, Gursoy S, et al. A case of squamous cell carcinoma of the thymus in a thymic cyst. Turkish Respiratory Journal 2007;8:110-2.
- Leong AS, Brown JH. Malignant transformation in a thymic cyst. Am J Surg Pathol 1984;8:471-5.
- Adachi T, Kawashima M, Aoshima H, Kijima H. A surgical case of thymic carcinoma arsisen in a thymic cyst. Journal of Japan Surgical Association. 2006;67:1257-60.
- Kobayashi T, Sumimoto T, Kohno N, Murakami E, Hiwada K, Takahashi H, et al. A case of squamous cell carcinoma of the thymus and thymic cyst. Nihon Kyobu Shikkan Gakkai Zasshi 1991;29:917-20. [Article in Japanese]
- Suster S, Rosai J. Cystic thymomas. A clinicopathologic study of ten cases. Cancer 1992;69:92-7.
- Leonidas JC, Berdon WE, Valderrama E, Neveling U, Schuval S, Weiss SJ, et al. Human immunodeficiency virus infection and multilocular thymic cysts. Radiology 1996;198:377-9.
- Truong LD, Mody DR, Cagle PT, Jackson-York GL, Schwartz MR, Wheeler TM. Thymic carcinoma. A clinico-pathologic study of 13 cases. Am J Surg Pathol 1990;14:151-66.


