Intralobar pulmonary sequestration in adults: three case reports
Abstract
Pulmonary sequestration is a relatively rare entity comprising a small portion of all congenital pulmonary malformations. Varying clinical techniques have been utilized to manage this disease process including abstaining from surgical intervention, endovascular procedures and operative approaches. We reviewed three case reports with varying presentations, each of which had a favorable outcome with definitive surgical treatment. In conclusion, we recommend that resection should continue to be the standard of care in both adolescent and adult patients with this disease process.
Key words: Pulmonary sequestration; intralobar; case report
Introduction
Pulmonary sequestration is a relatively rare entity comprising 0.15-6.4% of all congenital pulmonary malformations (1). Typically, it consists of a systemic arterial supply to an associated anomalous lung segment with various forms of venous drainage. These segments have no connection to the tracheobronchial tree. Sequestrations are typically divided into intralobar and extralobar forms, with the former being defined as a lung segment contained within the native pleural lining while the latter exhibits its own pleural investment.
Sixty percent of these lesions are diagnosed within the first decade of life and are more common in males by a 3:1 ratio (1). Symptoms may vary and typically are related to chronic respiratory infection although sequestrations may be discovered incidentally on radiographic studies. The arterial supply is variable with 74% being supplied by the thoracic aorta, while the remainder originate from the abdominal aorta and its branches including the gastric or splenic arteries (1,2). Typically, venous drainage from these lung segments is via the pulmonary venous system, although systemic drainage has been noted as well.
Attempts have been made to fit sequestrations into a continuum of congenital bronchovascular disorders which include primary parenchymal anomalies (for example, bronchial stenosis, bronchial cysts, and cystic adenomatoid malformation) as well as classic sequestrations and disorders of pulmonary venous drainage. Currently, the traditional term of “sequestration” is most commonly utilized to describe this entity.
Many theories have been suggested to elucidate the embryologic mechanism responsible for sequestrations. Rokitansky and Rektorzik received credit for making the first attempt in 1861 with their “Fraction Theory” which hypothesized that the sequestered segment is a separation of normally developed lung (3). More recently, others have hypothesized that during lung development, an insult to the developing pulmonary arterial blood supply occurs leading to retention and proliferation of the nascent systemic capillary network (4). In fact, the embryology of these defects remains uncertain.
We now demonstrate three recent cases of intralobar pulmonary sequestration at our institution with varying presentations.
Case report 1
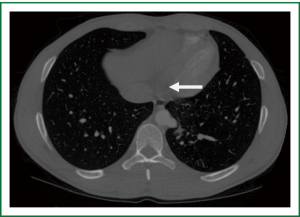
An eighteen year old athletic male with no significant past medical history presented with complaints of vague chest pain radiating to the right arm and associated shortness of breath. Laboratory values were normal with the exception of mild thrombocytopenia. Computed tomography (Figure 1) revealed an intralobar sequestration of the postero-basal segment of the left lower lobe, supplied by a solitary branch off of the thoracic aorta. The branch was eight centimeters superior to the esophageal hiatus and measured one centimeter in diameter. Venous return was to the left atrium and no alveolar opacification was noted to suggest pyogenic pneumonia. A dystrophic bronchial tree was identified that did not communicate with the normal bronchial tree. Of note, plain films of the chest were unremarkable. Preoperative pulmonary function tests demonstrated an FEV1 of 4.03 liters (95% predicted) and DLCO of 29.03 mL/min/mmHg CO (86% predicted).
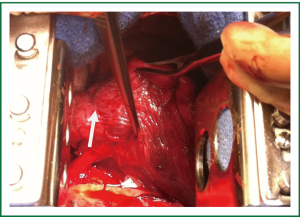
The patient was taken to the operating room where a standard posterolateral thoracotomy incision was made. The aberrant arterial supply was identified after mobilization of the inferior pulmonary ligament (Figure 2). Suture ligation was undertaken with pledgeted monofilament mattress sutures and the artery was then transected with the use of a vascular stapler. The remainder of the lobectomy was performed in the usual fashion.
The patient went on to have a relatively uncomplicated clinical course and was discharged on hospital day four in satisfactory condition. Pathologic review was notable for mild chronic inflammation along with overlying interstitial fibrosis and bronchial metaplasia. In addition, bronchial plugging along with an abundant arterial supply was identified to be consistent with intralobar pulmonary sequestration. The patient denied any complaints at follow-up and has continued to progress well.
Case report 2
A forty-seven year old female presented with a complaint of a chronic, dry cough and pneumonia that was recurrent in nature. Her past medical history was significant for Hashimoto’s thyroiditis requiring ablation therapy. Laboratory results revealed mild leukocytosis at 11.9 K/UL. Computed tomography was performed and a one centimeter diameter thoracic aortic branch was identified supplying an intralobar pulmonary sequestration of the left lower lobe. Active pneumonia was not demonstrated, however moderate mixed alveolar and interstitial opacities were present within the sequestration. Chest plain films did show an ill-defined density in the left lower lobe consistent with sequestration. Preoperative pulmonary function tests were essentially normal. The patient then underwent a left thoracotomy and left lower lobe resection, similar to that described above. Purulent material was visualized originating from the left lower lobe during the resection despite the institution of antibiotics 2 weeks prior to the operation. Her post-operative course was notable for a persistent air leak through hospital day four. This ultimately resolved and she was discharged following removal of the thoracostomy tubes. Pathologic review of the resected lung segment was pertinent for chronic and granulomatous inflammation along with interstitial fibrosis. She reported no complaints at follow-up.
Case report 3
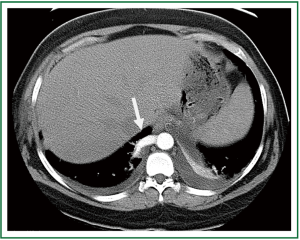
A twenty-five year old female presented with a history of multiple respiratory infections and intermittent right-sided chest pain. Her past medical history was significant for an arachnoid cyst along with migraine headaches. Prior surgeries included tonsillectomy, cholecystectomy and caesarean section. Laboratory results revealed mild hypokalemia and hypocalcemia without evidence of leukocytosis. Computed tomography revealed a 1.1 centimeter diameter right-sided descending aortic branch supplying the ipsilateral lower lobe (Figure 3). Diffuse alveolar infiltrates were noted over the posterior base without evidence of active pneumonia. Plain films of the chest did not show an active disease process within the thorax and preoperative bronchoscopy revealed chronic inflammation without other abnormalities.
The patient underwent a right lower lobectomy via a posterolateral thoracotomy in a fashion similar to the previous descriptions. Of note, chronic inflammation was again identified within the lower lobe while the remaining segments were free of gross abnormalities. Her postoperative course was remarkable for a persistent, small apical pneumothorax that was asymptomatic in nature. She was subsequently discharged home on post-operative day five without complications. Pathologic evaluation revealed marked intraalveolar hemorrhage without evidence of malignancy.
Discussion
Intralobar pulmonary sequestration is a relatively rare congenital anomaly with few reports of initial diagnosis occurring during adulthood. Patients can present with an incidental pulmonary lesion on imaging and be otherwise asymptomatic. More commonly however, they may manifest varying degrees of pulmonary symptomatology such as pleural effusions or recurrent pneumonia. Few reports have mentioned more severe sequelae such as overlying aspergillosis and even fatal hemoptysis (5-7). For these reasons sequestration has traditionally been treated by definitive resection of the affected lung segment.
Achieving a diagnosis of pulmonary sequestration can range in difficulty depending upon the type of anomaly and presenting symptoms. Computed tomography will typically suffice in most adult cases with some debate still held over the need for angiography (4). In our experience, the chest CT scan was clearly sufficient to make the diagnosis plus delineate the anatomic features notable for operative planning. For prenatal diagnosis, ultrasonography has become a useful tool, typically revealing a homogenous, echodense and well-defined mass (8). Despite its increasing utility, attempting to discriminate sequestration from other congenital malformations can prove to be difficult with the use of antenatal ultrasound alone. Specifically, cystic adenomatoid malformation and scimitar syndrome can mimic a sequestration (8,9), and can be difficult to differentiate. Postnatal ultrasound can lead to the diagnosis of scimitar syndrome by more clearly identifying normal lung echogenicity and a characteristic silhouette secondary to its abnormal venous drainage (9). As for cystic adenomatoid malformations, nuclear magnetic resonance imaging can aid in distinguishing the underlying pathology (8).
Lung cancer associated with sequestration in adults deserves special mention. There have been few reports of malignant neoplasms being involved in or near sequestered segments. In cases with simultaneous involvement, resection is obviously the mainstay of treatment. In contrast, if a patient has limited pulmonary reserve, it is recommended that the neoplasm be resected with the remainder of the lung and sequestered segment left in-tact to preserve as much lung function as possible (10). Another oncologic correlation worth discussing is the relationship between sequestration and elevated levels of CA19-9 and CA125. These tumor markers are often elevated in various forms of cancer including choledochal, pancreatic, lung, gastrointestinal and ovarian subtypes. Recent data has shown these can also be elevated in cases of benign lung disease including pulmonary sequestration and thus could be used as an additional diagnostic tool in the clinician’s armamentarium (11).
Definitive treatment involves resection of the affected lung segment. There are several key elements that should be considered: (I) a preoperative course of antibiotics in the setting of a pneumonia exacerbation can be beneficial by limiting the inflammation found at the time of surgery, (II) accurate preoperative identification of the arterial blood supply is crucial since inadvertent injury of these systemic vessels can have a fatal consequence (1), and (III) great care should be given to securing the systemic arterial branches at the time of operation, which can be quite large in diameter. The extent of resection is aimed at preserving as much normal lung tissue as possible thus warranting a sequestrectomy when feasible. This is more applicable when a diagnosis occurs during childhood due to the ability of further lung development in retained normal tissue. A lobectomy is appropriate in scenarios when it is difficult to distinguish sequestered tissue from functioning parenchyma (8).
Two alternative approaches should be noted. One is exclusion of the aberrant arterial supply via an endovascular approach, utilizing various occlusion devices. This carries the downside of retaining the un-aerated pulmonary parenchymal tissue that is still subject to recurrent infection (12). The other option is resection via minimal-access procedures such as VATS lobectomy (13). The advantages of this approach must be weighed against potential difficulty controlling the systemic arterial branches.
Conclusions
Pulmonary sequestration is a rare entity especially in the adult population. There can be a vast array of manifestations ranging from asymptomatic patients to those that present with sequelae, such as recurrent pulmonary infections or hemoptysis. Imaging studies are paramount in the diagnostic process with the modality dependent upon patient age and the differential diagnosis. In the adult, chest CT scan is most helpful, and will provide information about the vascular anatomy that is crucial for operative treatment. Management of sequestrations has ranged from observation in asymptomatic patients to varying degrees of interventional approaches in those with symptoms. Given the potential for recurrent infections and life-threatening hemoptysis in these patients, operative resection is the preferred approach. Three case reports were reviewed with differing presentations, each of which had a favorable outcome with definitive operative treatment. Early surgical resection should continue to be the standard of care in both adolescent and adult patients with this disease process.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Savic B, Bertel FJ, Tholen W, et al. Lung sequestration: report of seven cases and review of 540 published cases. Thorax 1979;34:96-101.
- Sellke F, Townsend C Jr, Beauchamp R, et al. Sabiston and Spencer’s surgery of the chest, eighth edition 2010.
- Halkic N, Cuénoud PF, Corthésy ME, et al. Pulmonary sequestration: A review of 26 cases. Eur J Cardiothorac Surg 1998;14:127-33.
- Clements BS, Warner JO. Pulmonary sequestration and related congenital bronchopulmonary-vascular malformations: nomenclature and classification based on anatomical and embryological considerations. Thorax 1987;42:401-8.
- Morikawa H, Tanaka T, Hamaji M, et al. A case of aspergillosis associated with intralobar pulmonary sequestration. Asian Cardiovasc Thorac Ann 2011;19:66-8.
- Somja J, De Leval L, Boniver J, et al. Intrapulmonary lung sequestration diagnosed in an adult. Rev Med Liege 2011;66:7-12.
- Rubin EM, Garcia H, Horowitz MD, et al. Fatal massive hemoptysis secondary to intralobar sequestration. Chest 1994;106;954-5.
- Andrade CF, Ferreira HP, Fischer GB. Congenital lung malformations. J Bras Pneumol 2011;37:259-71.
- Bhide A, Murphy D, Thilaganathan B, et al. Prenatal findings and differential diagnosis of scimitar syndrome and pulmonary sequestration. Ultrasound Obstet Gynecol 2010;35:398-404.
- Okamoto T, Masuya D, Nakashima T, et al. Successful treatment for lung cancer associated with pulmonary sequestration. Ann Thorac Surg 2005;80:2344-6.
- Yagyu H, Adachi H, Furukawa K, et al. Intralobar pulmonary sequestration presenting increased serum CA19-9 and CA 125. Intern Med 2002;41:875-8.
- Marine LM, Valdes FE, Mertens RM, et al. Endovascular treatment of symptomatic pulmonary sequestration. Ann Vasc surg 2011;25:696.e11-5.
- Gonzalez D, Garcia J, Fieira E, et al. Video-assisted thoracoscopic lobectomy in the treatment of intralobar pulmonary sequestration. Interact Cardiovasc Thorac Surg 2011;12:77-9.